Structural Molecular Biology
Illuminating Biological Structures at the Atomic and Molecular Levels



Structural Molecular Biology
The Structural Molecular Biology (SMB) Division and program enable scientists to rapidly obtain and utilize structural information on biomolecular and bioinspired systems at the atomic-to-micron scale, and at temporal resolutions, to understand function (and malfunction) in biological processes. Our goals range from innovative fundamental discovery-based science to applied uses, such as biotechnology, drug discovery, bioenergy and bioremediation. The SMB program has pioneered and will continue to lead development of new and enhanced techniques and facilities and make them widely and rapidly available to the biomedical, bioenergy, biogeochemical and environmental research communities.
The SMB program integrates macromolecular X-ray crystallography (MC), biological small/wide angle X-ray scattering/diffraction (SAXS/WAXS), µ-X-ray fluorescence (µXRF) imaging, and X-ray absorption (XAS) and emission spectroscopy (XES), across 8 beam lines, to holistically study the most challenging and wide-ranging biological systems.
We work closely with a broad community of scientists from academia, industry, and national laboratories in a commitment of collaboration, user support, training and education. At the core of the SMB program is a commitment to education of the future workforce through extensive outreach and training.
There is synergy with the LCLS X-ray free electron laser and the SLAC-Stanford cryo-electron microscopy (cryoEM) and cryo-electron tomography (cryoET) facilities and other advanced light and electron bioimaging programs across SLAC/SSRL. Collectively, this provides a remarkably rich and broad window on structure and function across a wide range of biologically relevant length and time scales, creating the foundation to extend results at the atomic and molecular level to understanding complex macromolecular interactions, and to studies of organelle, cell and tissue organization and function.
The SMB program engages with Stanford’s ChEM-H, with other user facilities and where relevant, lead multi-user-facility arrangements in areas that provide user access to complementary techniques, such as with the Environmental Molecular Science Laboratory (EMSL) and the Joint Genome Institute (JGI), and in a coordinated outreach program within BER-funded facilities and research groups.
SMB Programs
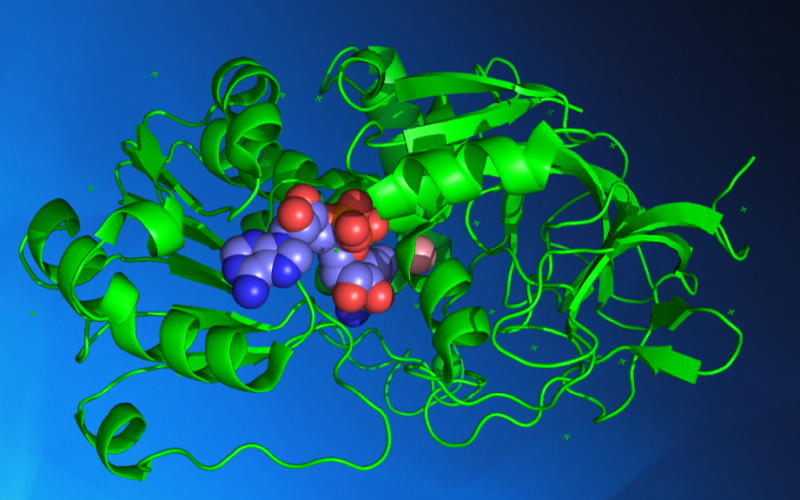
Macromolecular Crystallography
Macromolecular Crystallography is a technique used to study biological molecules such as proteins, viruses and nucleic acids to a spatial resolution at atomic level. This high resolution can provide the detailed mechanism by which these macromolecules carry out their functions in living cells and organisms.
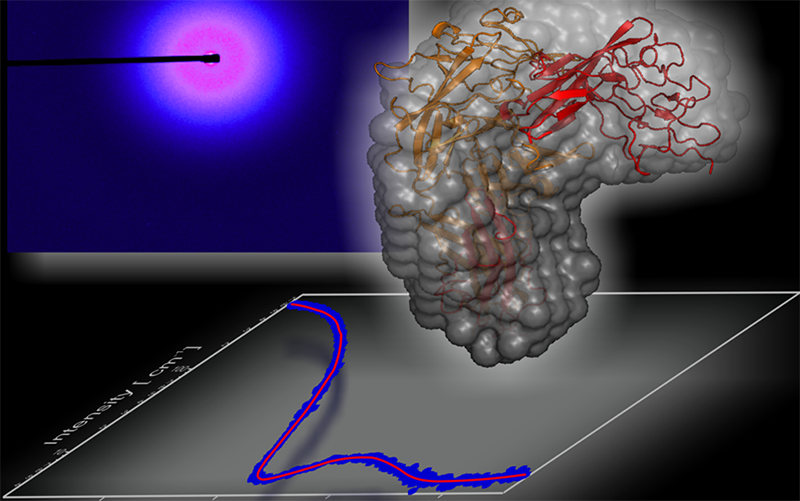
Biological Small Angle X-ray Scattering/Diffraction
Biological SAXS is a powerful probe for the structure and dynamics of proteins, nucleic acids, lipids and their complexes. It provides details at the molecular and nanoscale level in the physiologically relevant solution state of the biomolecules in action.
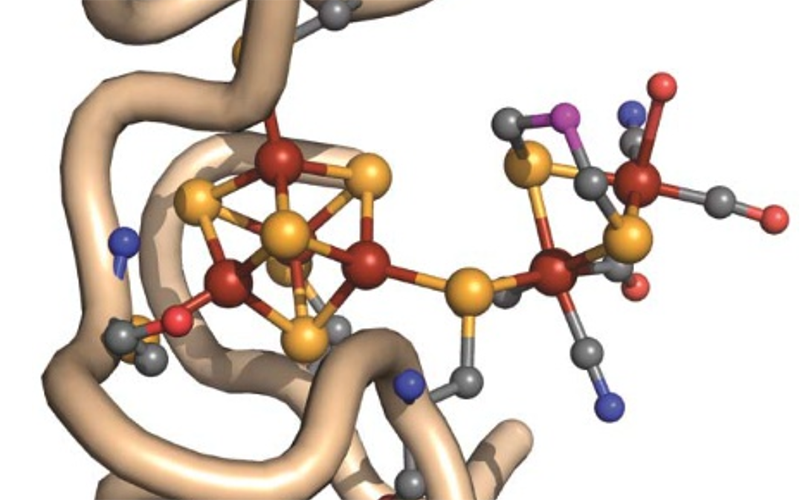
X-ray Absorption and Emission Spectroscopy
The SMB BioXAS program provides a combination of powerful spectroscopy and imaging facilities to unravel atomic resolution local structure, electronic properties, distribution and speciation of metal ions and ligands in biology accelerating biomedical and bioenergy breakthroughs.

Nobel Prizes
The 2006 Noble Prize in Chemistry was awarded to Roger Kornberg for research carried out in part at SSRL. Kornberg revealed the process of transcription, through which DNA's genetic blueprint directs the manufacture of proteins.
Due Dates
New Proposals & Extension Requests
| Run Period | X-Ray/VUV | Macromolecular Crystallography |
|---|---|---|
| Quarter 1 | May 1 | April 1 |
| Quarter 2 | August 1 | July 1 |
| Quarter 3 | November 1 | December 1 |
Beam Time Requests
| Run Period | X-Ray/VUV | Macromolecular Crystallography |
|---|---|---|
| Quarter 1 | August 1 | September 1 |
| Quarter 2 | November 1 | January 20 |
| Quarter 3 | February 22 | April 18 |
Partnerships & Collaborations
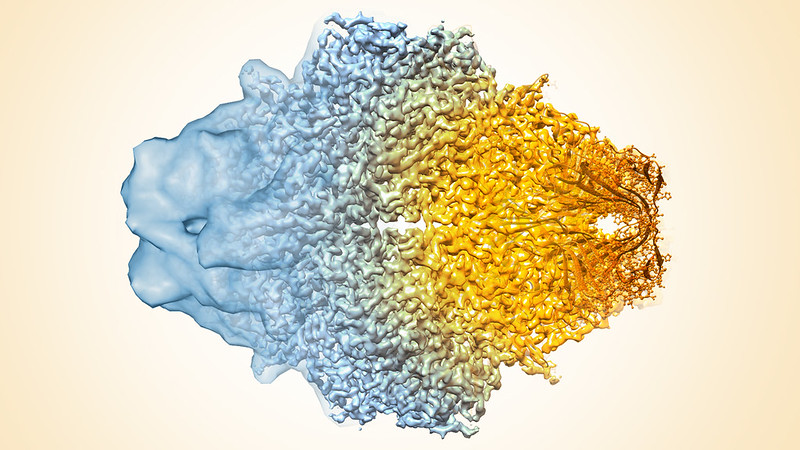
Cryo-EM (Cryogenic Electron Microscopy) Facility
Cryo-EM generates 3-D images at nearly atomic resolution of viruses, molecules and complex biological machines inside the cell, such as the ribosomes where proteins are synthesized. By flash-freezing these tiny things in their natural environments, scientists can see how they are built and what they do in much more detail than before, stringing thousands of images together to create stop-action movies and even taking virtual “slices” through cells, much like miniature CT scans.
SLAC Biosciences Division
The Biosciences Division builds on SLAC’s unique strengths in X-ray based research to explore biological function on multiple scales, from individual genes, proteins and enzymes to molecular ecosystems. SLAC’s world-class capabilities in ultrafast and high-throughput macromolecular crystallography, small-angle x-ray scattering, imaging and spectroscopy are optimal for revealing the physical and electronic structures of macromolecules in particular – the cornerstones of biological systems.

Multi-User Facility Collaborations
SSRL and LCLS scientists have partnered to develop the Macromolecular Femtosecond Crystallography (MFX) instrument at LCLS, home to a dedicated and highly automated goniometer-based setup for serial femtosecond crystallography experiments, with similar experimental hardware and control software as SSRL microfocus beam lines 12-1/2. The LCLS X-ray free electron laser expands the structural information accessible from small radiation-sensitive crystals, including metalloenzyme intermediate states. Applying short (fs) X-ray pulses, diffraction is obtained before substantial radiation-induced atomic rearrangements occur within the crystals, enabling ultra-fast time-resolved measurements.
Contacts & Resources
Aina Cohen
SMB Crystallography
(650) 926-3125
acohen@slac.stanford.edu
Michael Soltis
SMB Crystallography
(650) 926-3050
soltis@slac.stanford.edu
Thomas Weiss
SMB SAXS
(650) 926-5455
weiss@slac.stanford.edu
Ritimukta Sarangi
SMB XAS & Imaging
(650) 926-4621
ritis@slac.stanford.edu
Future Capabilities
| SMB Area | Diffraction | Scattering | Spectroscopy & Imaging | ||
|---|---|---|---|---|---|
| Macromolecular Crystallography | Micro-Beam, Undulator for Micro- to Nano-Crystallography | Micro- to Nano-Crystallography Instrumentation at LCLS | |||
| Biological SAXS | Microfocus Optics; High-Speed Detector | ||||
| Biological XAS and XES | New Detector Approaches | Advanced Spectroscopy Undulator (XES, XRS, RIXS, HERFD/XAS) | |||
| Picosecond to Femtosecond Time Domain | Micro- to Nano-Crystallography Instrumentation at LCLS | Advanced Spectroscopy Undulator (XES, XRS, RIXS) and LCLS | |||
