All living things require energy and an organism's ability to extract energy from its surroundings is a key requirement of survival. Until recently it was thought that there were only two methods to generate and conserve the energy required for cellular metabolism and survival. A consortium of researchers led by John Peters (Washington State University) revealed a third method of microbial energy production, called "flavin-based electron bifurcation" (FBEB). This method is completely different from the known processes, thus it represents a paradigm shift in how scientists think about the way organisms obtain energy.
The team characterized the flavin-based bifurcating enzyme NADH-dependent ferredoxin-NADP+ oxidoreductase I from Pyrococcus furiosus (Nfn) using x-ray crystallography in parallel with optical and electron paramagnetic resonance spectroscopies. Nfn catalyzes the formation of an energy-rich product, reduced ferredoxin, from the less energetic donor, NADPH, by coupling this unfavorable reaction to the thermodynamically favorable reduction of NADH by NADPH.
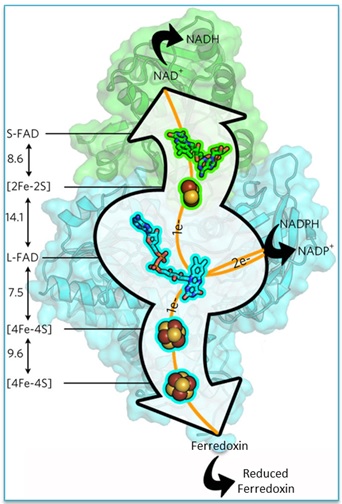
The structures of Nfn and NADPH bound to Nfn were solved to high resolution (~1.6 Å) using data collected on SSRL Beam Line 12-2. The initial Nfn structure was solved by single wavelength anomalous dispersion (SAD) using iron, tantalum and platinum for anomalous scatterers. The Nfn structure is composed of two subunits that harbor two cofactor chains that form the bifurcated electron transfer pathways from a central flavin adenine dinucleotide (L-FAD in Fig. 1).
The exothermic NADPH-NADH half reaction in Nfn is presumed to be mediated by NADPH oxidation at the L-FAD followed by electron transfer to a proximal [2Fe-2S] cluster and subsequently to the NADH binding site at a distal S-FAD cofactor (Fig. 2A). The [2Fe-2S] cluster is unusual with a relatively high positive reduction potential and coordination by one Asp and three Cys ligands. The S-FAD cofactor forms a neutral semiquinone stabilized by the hydrogen bonding and protein interactions at the N5 position of the isoalloxazine ring.

Figure 2. Two coupled electron-transfer pathways in the Nfn structure: (A) the exothermic pathway involves cofactors (L-FAD, [2Fe-2S] cluster and S-FAD) and (B) the endothermic involves L-FAD and two [4Fe-4S] clusters. Key residues at the bifurcating cofactor L-FAD site contribute to control reactivity.
The coupled endothermic half reaction between NADPH and ferredoxin is mediated by electron transfer through two [4Fe-4S] clusters: a proximal cluster located near the L-FAD and a distal cluster close to the protein surface where ferredoxin binds (Fig. 2B). The formation of an unstable anionic semiquinone (observed spectroscopically) is facilitated by the structure around the N5 position of the L-FAD; an Arg residue that is within hydrogen bonding distance of N5 appears to control the protonation state of the L-FAD. The researchers hypothesize that the hydrogen bond between Arg and N5 obstructs protonation of the L-FAD semiquinone, preventing formation of a lower-energy intermediate. It is the formation of this highly energetic intermediate that allows the thermodynamically unfavorable reduction of ferredoxin.
The detailed mechanism of FBEB revealed in this work provides a fundamental basis and structural framework for understanding how bifurcating enzymes have evolved to accomplish demanding biochemical reactions with energy conservation. This knowledge also presents an opportunity for the design of synthetic enzymes that can utilize a highly-energetic anionic semiquinone intermediate to perform thermodynamically unfavorable catalysis. It may also be possible to take advantage of these energy-efficient pathways inside living cells by engineering microbes to preferentially use them to make better products such as chemicals, fuels, or hydrogen gas.
C. E. Lubner, D. P. Jennings, D. W. Mulder, G. J. Schut, O. A. Zadvornyy, J. P. Hoben, M. Tokmina-Lukaszewska, L. Berry, D. M. Nguyen, G. L. Lipscomb, B. Bothner, A. K. Jones, A.-F. Miller, P. W. King, M. W. W. Adams and J. W. Peters, "Mechanistic Insights into Energy Conservation by Flavin-based Electron Bifurcation", Nat. Chem. Biol. 13, 655 (2017), doi: 10.1038/nchembio.2348

