Cells of the immune system communicate messages between them via small secreted complexes called cytokines. Cytokines are recognized by other cells through membrane receptors. These cell signaling pathways convey information about pathogens, cancers, or other problems that concern the immune system. Two of these cytokines, interleukin 12 (IL-12) and Interleukin 23 (IL-23) are made by antigen-presenting cells and help to activate lymphocytes, including both T and NK cells.
IL-12 and IL-23 have similar structures, which include a four-helix bundle α-subunit and a β-subunit called p40. Despite intense basic research and clinical interest in IL-12 and IL-23, a structural basis for receptor assembly has remained elusive.
In work led by the Garcia group at Stanford University, researchers determined the crystal structure of the complete IL-23 receptor complex using x-ray crystallography data collected on SSRL BL12-2.
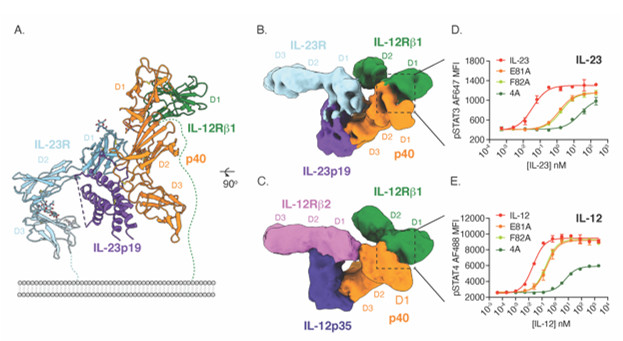
The structure revealed that IL-23 uses the four-helix bundle and p40 subunits to engage its’ receptors in a modular fashion (Fig. 1A). To extend these findings to the related cytokine IL-12, the group used yeast surface display to increase the affinity of IL-12Rβ1 for cytokine binding. With this high affinity receptor, the authors were able to stabilize IL-12 and IL-23 receptor complexes for cryoEM analysis in collaboration with Prof. Georgios Skiniotis (Stanford University). The resulting cryoEM maps show that both IL-12 and IL-23 engage the shared IL-12Rβ1 receptor via a common p40 subunit of the cytokine, while specific receptors IL-12Rβ2 and IL-23R bind the four-helix bundle component (Fig. 1B-C). The shared nature of the p40 subunit enabled the authors to design mutated versions of IL-12 and IL-23 that modulate downstream signaling of STAT3 and STAT4 (Fig. 1D-E). Using this strategy, the authors engineered versions of IL-12 that stimulate T cells with reduced activity on NK cells (Fig. 2A-B). In vivo, these T cell biased IL-12 agonists promoted anti-tumor immunity without inducing toxicity associated with NK cell activation (Fig. 2C-D).
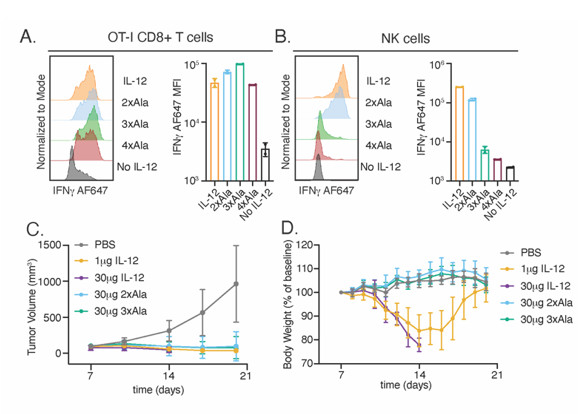
Figure 2. Design of T cell biased IL-12 agonists promotes anti-tumor immunity without NK cell mediated toxicity. (A-B) Characterization of IL-12 agonists in vitro. (A) Ovalbumin-specific OT-I T cells were stimulated with several variants for 48 hours prior to analysis of IFNγ by flow cytometry. (B) NK cells were stimulated with IL-18 and 1µM IL-12 variants for 48 hours. (C-D) IL-12 agonists support anti-tumor immunity without toxicity. (C) Tumor volumes of cytokine and control treated mice. (D) Body weight measurements of tumor bearing mice.
The use of cytokine partial agonists to increase cell-type selectivity present a path forward for the clinical development of cytokines which have previously not been viable due to pleotropic effects on multiple cell types.
C. Glassman, Y. K. Mathiharan, K. M. Jude, L. Su, O. Panova, P. J. Lupardus, J. B. Spangler, L. K. Ely, C. Thomas, G. Skiniotis and K. C. Garcia, "Structural Basis for IL-12 and IL-23 Receptor Sharing Reveals a Gateway for Shaping Actions on T versus NK Cells", Cell 184, 983 (2021) doi: 10.1016/j.cell.2021.01.018

