Type A γ-aminobutyric acid receptors (GABAARs) control neuronal excitability1. They are targets for the treatment of neurological diseases and disorders and also for general anesthetics. The underlying mechanisms of these drugs’ action on GABAARs remain to be determined.
One of the mechanisms is to potentiate function of GABAARs via binding to the transmembrane domain (TMD)2. Ample experimental evidence suggests that the TMD of GABAARs harbors sites for the primary actions of general anesthetics and neurosteroids. The TMD plays an essential role in functional transitions among the resting, activated, and desensitized states of these Cl--conducting channels.
Alphaxalone (5α-pregnan-3α-ol-11,20 dione) is a potent neurosteroid anesthetic. The anxiolytic, anticonvulsant, analgesic, and sedative-hypnotic effects of alphaxalone have been linked to its potentiation of GABA-evoked currents and direct activation of GABAARs3. However, the data about the alphaxalone binding site in GABAARs and the underlying structural basis of alphaxalone’s action are sparse.
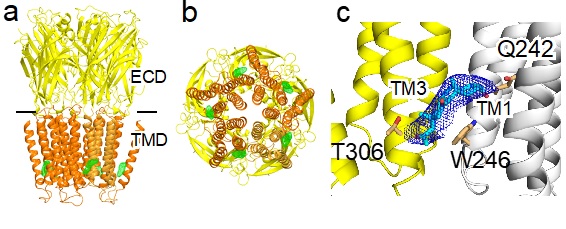
Figure 1. Crystal structures of the α1GABAAR chimera in complex with alphaxalone (a) Side and (b) bottom views of the structure. The extracellular domain (ECD and transmembrane domain (TMD) are colored in yellow and orange, respectively. Alphaxalone binding to the inter-subunit sites in the TMD is indicated by the FO-FC omit electron density map contoured at 4 σ (green mesh). (c) A zoom-in view of alphaxalone (cyan) bound to a pocket lined by residues in the TMD from the principal (yellow) and complementary (white) subunits of the α1GABAAR chimera. Alphaxalone is surrounded by the 2FO-FC electron density map contoured at 1 σ (blue mesh). Three residues in close contact with alphaxalone are highlighted.
In a recent paper published in Nature Communications (1), scientists from the University of Pittsburgh School of Medicine in collaboration with scientists from SSRL have successfully co-crystallized alphaxalone with an α1GABAAR chimera. Their crystal structures (Figure 1) illustrate the molecular details of alphaxalone binding to the α1GABAAR TMD and reveal neurosteroid anesthetic action starting at the bottom of the TMD.
Additionally, the study finds that alphaxalone allosterically triggers the activation and subsequent desensitization of the α1GABAAR chimera (1). More importantly, a comparison of apo and alphaxalone-bound crystal structures reveals a set of conformational changes that suggest a plausible path for alphaxalone-induced channel activation and desensitization, starting at the bottom of the TMD (Fig. 2).
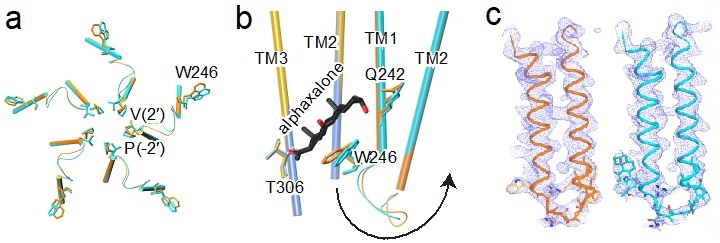
Figure 2. Alphaxalone-induced structural changes at the bottom of the TMD (a) Bottom view of overlaid TM1-TM2 structures of the apo (orange) and alphaxalone-bound (cyan) α1GABAAR chimera. (b) Side view of overlaid structures of apo (principal subunit – gold; complementary subunit - orange) and alphaxalone-bound (principal subunit – blue; complementary subunit - cyan) α1GABAAR chimera. For clarity, only TM2 and TM3 are shown in the principal subunit and only TM1 and TM2 are shown in the complementary subunit. The arrow highlights structural perturbations originating from the alphaxalone binding site near W246 through the TM1-TM2 linker to the pore-lining residues P253 (-2′) and V257 (2′). (c) The 2FO-FC electron density maps (blue mesh, contoured at 1 σ) covering TM1-TM2 in the apo (left) and alphaxalone-bound (right) α1GABAAR chimera. The sidechains are shown only for residues W246 to V257 (2′).
Together, the studies underscore three conclusions. First, alphaxalone binding to the inter-subunit site at the bottom of the TMD can introduce global conformational changes involved in channel potentiation, activation, and desensitization. Second, the TM1-TM2 linker, noted previously for its involvement in channel desensitization, plays a key role in mediating activation and potentiation by neurosteroids. Finally, this mechanism of activation or potentiation starting at the bottom of the TMD can be exploited in the rational search for new GABAAR modulators with better potency and efficacy.
- M. Farrant and Z. Nusser, “Variations on an Inhibitory Theme: Phasic and Tonic Activation of GABAA Receptors”, Nat. Rev. Neurosci. 6, 215 (2005)
- R. Jurd, M. Arras, S. Lambert, B. Drexler, R. Siegwart, F. Crestani, M. Zaugg, K. E. Vogt, B. Ledermann, B. Antkowiak and U. Rudolph, “General Anesthetic Actions in Vivo Strongly Attenuated by a Point Mutation in the GABAA Receptor Beta3 Subunit”, FASEB J. 17, 250 (2003)
- G. A. Cottrell, J. J. Lambert and J. A. Peters,”Modulation of GABAA Receptor Activity by Alphaxalone", Br. J. Pharmacol. 90, 491 (1987)
Q. Chen, M. M. Wells, P. Arjunan, T. S. Tillman, A. E. Cohen, Y. Xu and P. Tang, "Structural Basis of Neurosteroid Anesthetic Action on GABAA Receptors", Nat. Commun. 9, 3972 (2018) doi: 10.1038/s41467-018-06361-4

