The toxicity of arsenic is widely known, but perhaps less widely appreciated is that it's the level of toxicity critically depends on the chemical form. The fern Pteris vittata, is one of a small group of plants that actively accumulates to a startling degree - an arsenic hyperaccumuatlor. P. vittata absorbs arsenic from soil, typically present as the relatively benign arsenate, and changes its chemical form to arsenite, which is one of the more toxic kinds of arsenic. The plant thrives on this toxic regimen, and it most likely does this to defends itself from hungry herbivores. The ability of P. vittata to take up arsenic has generated much excitement because of potential applications for environmental cleanup of drinking water and of contaminated sites.
Approximately 1,700 scientists visit SSRL annually to conduct experiments in broad disciplines including life sciences, materials, environmental science, and accelerator physics. Science highlights featured here and in our monthly newsletter, Headlines, increase the visibility of user science as well as the important contribution of SSRL in facilitating basic and applied scientific research. Many of these scientific highlights have been included in reports to funding agencies and have been picked up by other media. Users are strongly encouraged to contact us when exciting results are about to be published. We can work with users and the SLAC Office of Communication to develop the story and to communicate user research findings to a much broader audience. Visit SSRL Publications for a list of the hundreds of SSRL-related scientific papers published annually. Contact us to add your most recent publications to this collection.
Genes, which are made of nucleic acids (DNA or RNA) contain the instructions for how to make proteins, but still enzymes made of proteins are needed to replGenes, which are made of nucleic acids (DNA or RNA) contain the instructions for how to make proteins, but still enzymes made of proteins are needed to replicate the genes. This paradox was addressed ~20 years ago with the realization that some kinds of RNA can act as enzymes. These RNA enzymes, or ribozymes, are accordingly made of the genetic RNA material, but they act as chemical catalysts. This means that ribozymes would have enabled the first self-replicating molecules, also made of RNA, to copy themselves.
Chemists have synthesized and characterized a new, highly reactive form of iron that promises to deepen our understanding of this important element. Iron is found in abundance in the natural world, and in its ionized form plays a crucial role in virtually all living processes.
Porous nanoscale materials often have useful properties because of their proportionally large surface areas. Now, UCLA scientists have devised a way to make porous germanium, a semiconductor used in fiber optics and electrical components. This discovery means that nanoporous materials could soon be used to develop new kinds of solar cells or highly sensitive electronic sensors.
After years of wondering how organisms managed to create medically valuable natural products, like antibiotics and anti-fungal agents, chemists have discovered the surprisingly simple secret by shining x-ray light on the problem. MIT and Harvard researchers used crystallography beam lines at the Stanford Synchrotron Radiation Laboratory and the Advanced Light Source in Berkeley for their research.
Researchers from the City of Hope cancer research and treatment center in Duarte, California, determined the crystal structure of the protein that controls this defense system in bacteria called Bacillus caldolyticus. Unless stopped, viral DNA slips into bacterial DNA, where it gets copied many times over, and then destroys its host. To protect bacterial cells, the control protein ensures the proper ratio between two enzymes, the "swords" and the "shields." The sword enzyme slashes invading viral DNA into useless pieces. The shield enzyme adds a protective layer to bacterial DNA, so the sword will not cut its master. Too few shields lead to bacterial cell death, and too many shields protect the viral DNA as well.
X-ray crystallography studies at the Stanford Synchrotron Radiation Laboratory recently shone light on a human enzyme that helps synthesize heme, the iron-containing pigment that helps carry oxygen to all parts of our bodies. There are many enzymes along the chemical pathway that produces heme. Defects in any one of the enzymes cause different types of porphyria, a set of symptoms that includes acute pain, neurological problems, and even the madness suffered by King George III.
Stanford Synchrotron Radiation Laboratory (SSRL) and Stanford researchers have now shown that the electrical performance of plastic semiconductors can be controlled and improved with surface treatments. In their research, published in Nature Materials, they showed they could align the small crystals within the polymer by applying a thin layer of another kind of organic molecule on to the surface. The highly-oriented crystals give the material better performance in conducting electricity. Researchers used x-ray scattering facilities at SSRL to determine the orientation of the crystals.
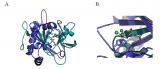
In rheumatoid arthritis and Crohn's disease, the immune system overreacts, provoking too much inflammation. One method of treatment is to inhibit the immune protein that incites inflammation, called tumor necrosis factor (TNF). Currently available anti-TNF therapeutics have made a significant difference to patients, but are costly to manufacture and require an I.V. or injection. Sunesis Pharmaceuticals of South San Francisco, in collaboration with Biogen Idec, is researching small molecules that will inhibit TNF. The advantage of using small molecules is that they can be administered orally, and be produced much less expensively.
Gene therapy can potentially cure many hereditary and acquired diseases, such as cancer, hemophilia and cystic fibrosis, by delivering a healthy copy of a gene to the cells that need it. Researchers have been working on ways to deliver genes safely and effectively to the right locations. One promising approach is to use negatively charged lipids that reside in cell membranes of mammals. The idea is to pack a gene, made of DNA, into a lipid pocket, which then fuses with a cell membrane and empties the gene into the cell. The advantage of these anionic lipids (AL) is they do not evoke an immune response. The disadvantage is they do not attach well to DNA because both are negatively charged.