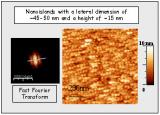
New approaches to the fabrication of ferroelectric nanostructures onto substrates are critical for the development of competitive functional devices that successfully integrate at nanoscale ferroelectrics as alternative materials in the microelectronic industry. These approaches have to meet reliability and utilization requirements to realize a cost-effective production of an increasing demand for ultra-high-density memories or nanometric electromechanical systems. An important challenge in the fabrication of ferroelectric nanomaterials supported onto substrates is the ability to fabricate an organized arrangement of the nanostructures. This is a key point for the applications of ferroelectrics in nanoelectronic devices.
Approximately 1,700 scientists visit SSRL annually to conduct experiments in broad disciplines including life sciences, materials, environmental science, and accelerator physics. Science highlights featured here and in our monthly newsletter, Headlines, increase the visibility of user science as well as the important contribution of SSRL in facilitating basic and applied scientific research. Many of these scientific highlights have been included in reports to funding agencies and have been picked up by other media. Users are strongly encouraged to contact us when exciting results are about to be published. We can work with users and the SLAC Office of Communication to develop the story and to communicate user research findings to a much broader audience. Visit SSRL Publications for a list of the hundreds of SSRL-related scientific papers published annually. Contact us to add your most recent publications to this collection.
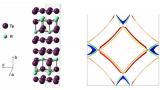
A collaboration between scientists at SSRL and the department of Applied Physics at Stanford University has determined the phase diagram of a new family of prototypical charge density wave (CDW) compounds. These compounds have the chemical formula RTe3, where R represents a rare earth element from La to Tm. In research, the collaborators have used X-ray diffraction and resistivity measurements to determine the factors affecting the symmetry of the CDW state, specifically whether the CDW runs in one direction or two.
The development of bacterial resistance to conventional antibiotics is a major public health concern. For example, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE) and Staphylococcus aureus (VRSA) have emerged as common nosocomial (hospital-originating) infections. Circumvention of such resistance may be possi ble by emulating host defense antimicrobial peptides (AMP's), which are found in a broad range of species and have broad-spectrum antimicrobial properties. These AMP's have two structural motifs in common: they are cationic and amphipathic. It is thought that electro static interactions facilitate association of the peptide with the anionic bacterial membrane and amphiphilic interactions act to form pores in the bacterial membrane, leading to cell death.
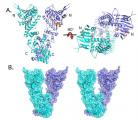
Proteins are transported to specific sites within cells enclosed in packets called transport vesicles, along a specialized network of tracks called microtubules. Transport vesicles are targeted to the correct acceptor membrane by a number of sequential steps that are regulated by small GTPases of the Rab and Arf families. The initial interaction between vesicles and the target membrane is thought to be mediated by very large molecular "tethers" that link the two membranes prior to fusion. A Stanford team from the Brunger and Pfeffer laboratories has studied how one such putative tether molecule is localized to the membranes of an organelle called the Golgi complex.
Life depends on the biochemical activity of the thousands of proteins that inhabit and decorate the surface of every one of our cells. Proteins themselves, although simple linear combinations of the twenty amino acids, derive their remarkable properties from the complex three-dimensional structures into which they fold. In this way, enzyme active sites are created, protein-protein recognition surfaces are formed, and the chemistry of life is set in motion.
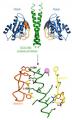
Autism is a neurodevelopmental disorder that impairs social interactions, and causes communication deficits and repetitive behaviors. About 1 in every 150 children is affected by autism. Genetic screens revealed that mutations in the neurexin and neuroligin genes are among the multiple genetic causes of autism spectrum disorders and mental retardation (Jamain et al., 2003; Szatmari et al., 2007). In the brain, neurexins and neuroligins are cell adhesion proteins on the pre-synaptic and post-synaptic cell membranes, respectively. Their extracellular domains interact with each other within the synaptic cleft to provide connectivity between nerve cells and assure proper synapse function.
A team of scientists, working in part at SSRL's crystallography beam lines and led by Stanford Professor Roger Kornberg, has determined for the first time the atomic structure (at 1.1 Å resolution) of a thiol-covered gold nanoparticle, a discovery with potential for a range of applications from biosensors to nanotransistors. The results are published in the October 19 issue of Science.
Since the discovery of high-temperature superconductor by Bednorz and Müller in 1986, this field has become one of the most important research topics in solid state physics. In the past 20 years many unconventional properties have been discovered in this new class of materials. These have challenged our conventional wisdom and driven the development of many novel theories. Among these discoveries, the most mysterious is probably the pseudogap phenomena: it has been observed that there is an energy gap above the superconducting transition temperature (TC) that persists over a wide range of temperatures and chemical compositions [1]. This peculiar behavior appears to be very different from a conventional superconductor. Here the electrons form so-called "Cooper pairs", which manifests itself as an energy gap in many spectroscopic measurements.
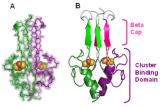
Proteins containing iron-sulfur clusters are ubiquitous in nature and catalyze one-electron transfer processes. These proteins have evolved into two classes that have large differences in their electrochemical potentials: high potential iron-sulfur proteins (HiPIPs) and bacterial ferredoxins (Fds). The role of the surrounding protein environment in tuning these redox potentials has been a persistent puzzle in the understanding of biological electron transfer. Although high potential iron-sulfur proteins and ferredoxins have the same iron-sulfur structural motif, there are large differences in their electrochemical potentials.
The rise in obesity in the United States parallels a dramatic increase in obesity-associated diseases, most notably type-2 diabetes. This disease is predicted to reach epidemic proportions in the next several decades (Zimmet et al 2001, Urek et al 2007). Thus, understanding the biochemical processes underlying type-2 diabetes and identifying new targets for therapeutic intervention are critical for national and world health. A drug of choice to treat type-II diabetes is pioglitazone, a thiazolidinedione (TZD) derivative originally thought to exert its effect through activation of the nuclear transcription factor PPARg. Recently, a novel protein target for pioglitazone was discovered and was called mitoNEET (Colca et al 2004). This protein is anchored to the outer mitochondrial membrane (OMM) (Wiley et al 2007).