Though life on earth is composed of a diverse range of organisms, some with many different types of tissues and cells, all these are encoded by a molecule we call DNA. The information required to build a protein is stored in DNA within the cells. Not all the message in the DNA is used in each cell and not all the message is used all the time. During cell differentiation, the cells become dedicated for their specific function which involves selectively activating some genes and repressing others. Gene regulation is an important event in the developmental biology and the biology of various diseases, but a more complex process.
In the bacteria there are distinct enzymes while one is capable of cleaving DNA, the other protects DNA by modification. The complementary function provided by the set of enzymes offers a defense mechanism against the phage infection and DNA invasion. The incoming DNA is cleaved sequence specifically by the class of enzymes called restriction endonuclease (REase). The host DNA is protected by the sequence specific action of matching set of enzymes called the DNA methyltransferase (MTase). The control of the relative activities of the REase and MTase is critical because a reduced ratio of MTase/REase activity would lead to cell death via autorestriction. However too high a ratio would fail to provide protection against invading viral DNA. In addition a separate group of proteins capable of controlling R-M proteins have been identified in various restriction-modification (R-M) systems which are called C proteins (Roberts et al., 2003).
A homolog of R-M C protein, named as C.BclI regulates the expression level of M.BclI (methyltransferase) in E. coli differently. In the absence of C.BclI, M.BclI was over-expressed and displayed relaxed specificity for methylation of non-cognate (non-specific or less specific) sites. However, in the presence of C.BclI and C box (the C.BclI binding sequence), the expression level of M.BclI was down regulated and M.BclI did not show relaxed specificity.
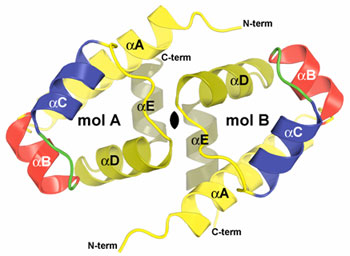
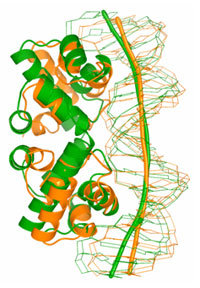
Balendiran's group at City of Hope collected the x-ray diffraction data on BL1-5 at SSRL. The crystal structure of C.Bcll was determined to 1.8 Å resolution by anomalous dispersion methods, using mercury derivatives. The high-resolution crystal structure of C.BclI uncovers the presence of a helix-turn-helix (HTH) motif (Figure 1) and the potential to bend B-DNA around the C.BclI dimer (Figure 2). Furthermore interactions with DNA are likely mediated by residues on the surface of the HTH motif helices between 34 and 48 in the structure. Moreover by analogy to known proteins with HTH motif (Hochschild et al., 1983; Bushman and Ptashne, 1988; Bushman et al., 1989) highly conserved residue Glu27 in the C family of proteins and partly conserved Asp31 residue in C.BclI may play a crucial role in the interaction with RNA polymerase and be important for transcriptional control.
Acknowledgement
The study was conducted in collaboration with Shuang-yong Xu, Ph.D., senior scientist, New England Biolabs, with assistance from Professor David Eisenberg, Professor Richard Dickerson, Duilio Cascio Ph.D., Michael R. Sawaya, Ph.D., University of California at Los Angeles; Stanford Synchrotron Radiation Laboratory and SSRL staff; Jim D'Aoust, Project Manager, Cyberinfrastructure Partnership, San Diego Supercomputer Center; Richard Roberts, Ph.D., CSO, Nobel laureate, and Elisabeth Raleigh Ph.D., research director, New England Biolabs Inc.
- Bushman, F. D., and Ptashne, M. (1988). Turning lambda Cro into a transcriptional activator. Cell 54, 191-197.
- Bushman, F. D., Shang, C., and Ptashne, M. (1989). A single glutamic acid residue plays a key role in the transcriptional activation function of lambda repressor. Cell 58, 1163-1171.
- Hochschild, A., Irwin, N., and Ptashne, M. (1983). Repressor structure and the mechanism of positive control. Cell 32, 319-325.
- Roberts, R. J., Belfort, M., Bestor, T., Bhagwat, A. S., Bickle, T. A., Bitinaite, J., Blumenthal, R. M., Degtyarev, S., Dryden, D. T., Dybvig, K., et al. (2003). A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes.Nucleic Acids Res 31, 1805-1812
Sawaya, M. R. Zhu, Z., Mersha, F., Chan, S-h., Dabur, R., Xu, S-y., Balendiran, G. K., Crystal structure of the restriction-modification system control element C.BclI and mapping of its binding site. Structure 13:1837-1847, (2005).




