Iron is the most abundant transition element on earth, and is typically found in formal oxidation states of either II or III. However, high valent Fe(IV) and Fe(V) complexes are invoked in the mechanisms of both heme and non-heme enzymes; and Fe(VI) is known to exist in the mineral ferrate.[1] Ferrate is a powerful oxidant, which has been used in soil and wastewater treatment, batteries, and disinfectants; however, it is unstable and often indiscriminately reactive. This has driven chemists to try and synthesize other hexavalent iron species, but until recently this was not possible. In the June 30 issue of Science, John Berry and co-workers reported the synthesis and characterization of the second known compound of Fe(VI) [2].
Using an Fe(IV)-azide(Me3-cyclam-acetato) complex, an Fe(VI) species can be generated by photolysis with 650 nm light, producing a formally Fe(VI)-nitrido complex. This photolysis product has been investigated through detailed spectroscopic studies, combined with DFT calculations. X-ray absorption spectroscopic measurements (conducted at SSRL BL9-3) were key in establishing that this complex is a genuine Fe(VI) complex with a very short (1.57 Å) Fe-N(nitrido) bond. The XAS edge energy increases as the effective nuclear charge on the Fe complex increases. This results in an ~1 eV shift in edge energy for the Fe(VI) complex, as compared to a previously reported Fe(V) complex (Figure 1) [3]. The EXAFS data provide local structural information, providing an experimental measure of the Fe-N(nitrido) bond length.
Figure 1 (below) shows a comparison of the normalized Fe K-edge XAS data of the Fe(VI)-nitrido complex to that of a previously reported Fe(V)-nitrido complex.[3] There is an intense pre-edge transition feature in the energy range 7114-7115 eV in each case, consistent with the presence of a highly covalent iron-ligand multiple bond. The area under this pre-edge transition is significantly higher than the reported area for any Fe(IV)-oxo species, reflecting a greater covalency of the Fe≡N bond in the Fe(VI)-nitrido complex.[4] In addition, the pre-edge and rising edge of the Fe(VI) complex is shifted to ~1 eV higher energy than the Fe(V), consistent with an increase of one unit in oxidation state.
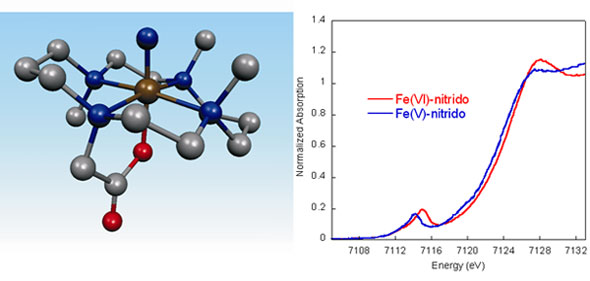
The EXAFS data provide local structural information on the Fe(VI) complex, which could not be obtained from any other method, as this complex is generated only at low concentrations (~1 mM in Fe) in solution. The EXAFS data are best fit with one short Fe-N bond length of 1.57 Å and an average of five Fe-N or Fe-O bond distances of 2.03 Å. The 1.57 Å Fe-N bond length is fully consistent with the description of this interaction as a metal-ligand triple bond. DFT calculations further support this result. The proposed structure of the Fe(VI) complex, based on EXAFS and DFT results, is shown in Figure 1 (left).
The reported Fe(VI)-nitrido complex is to our knowledge the first example of an octahedral coordination complex of Fe(VI). The complex differs significantly from the tetrahedrally coordinated Fe(VI) in ferrate. These differences in geometric and electronic structure should result in differences in reactivity, which are currently being investigated. In addition, the results of these experiments provide important spectroscopic markers for high-valent Fe species and should encourage the consideration of high valent intermediates in biological systems, where our proposals are often dictated by what can be synthesized in small molecule systems.
- L. D. Slep, F. Neese, Angew. Chem. Int. Ed. 2003, 42, 2942 and references therein. b) M. Costas, M. P. Mehn, M. P. Jensen, L. Que Jr., Chem. Rev. 2004, 104, 939; c) D. L. Harris, Curr. Op. Chem. Biol. 2001, 5, 724.
- J. F. Berry, E. Bill, E. Bothe, S. DeBeer George, B. Mienert, B. F. Neese, K. Wieghardt,Science 2006, 312, 1937.
- N. Aliaga-Alcalde, S. DeBeer George, B. Mienert, E. Bill, K. Wieghardt, F. Neese, Angew. Chem. Int. Ed. 2005, 44, 2908.
J. F. Berry, E. Bill, E. Bothe, S. DeBeer George, B. Mienert, B. F. Neese, K. Wieghardt, Science 2006, 312, 1937.




