X-ray free-electron lasers (XFELs) will produce photon pulses with a unique and desirable combination of properties. Their short X-ray wavelengths allow penetration into materials and the ability to probe structure at and below the nanometer scale. Their ultra-short duration gives information about this structure at the fundamental time-scales of atoms and molecules. The extreme intensity of the pulses will allow this information to be acquired in a single shot, so that these studies can be carried out on non-repeatable processes or on weakly-scattering objects that will be modified by the pulse. A fourth property of XFEL pulses is their high transverse coherence, which brings the promise of decades of innovation in visible optics to the X-ray regime, such as holography, interferometry, and laser-based imaging.
Approximately 1,700 scientists visit SSRL annually to conduct experiments in broad disciplines including life sciences, materials, environmental science, and accelerator physics. Science highlights featured here and in our monthly newsletter, Headlines, increase the visibility of user science as well as the important contribution of SSRL in facilitating basic and applied scientific research. Many of these scientific highlights have been included in reports to funding agencies and have been picked up by other media. Users are strongly encouraged to contact us when exciting results are about to be published. We can work with users and the SLAC Office of Communication to develop the story and to communicate user research findings to a much broader audience. Visit SSRL Publications for a list of the hundreds of SSRL-related scientific papers published annually. Contact us to add your most recent publications to this collection.
Autism is considered among the most devastating neurological disorder conditions of early childhood. Now, researchers working in part at SSRL's Beam Line 4-2 have determined a three-dimensional structural model of a complex with the only two extracellular synaptic proteins implicated in autism spectrum disorders and mental retardation. Such a finding could deepen our understanding of this mysterious and debilitating type of disorder. The findings were published in the June 2007 edition of the journal Structure.
Mercury toxicity generates environmental concerns in diverse aquatic systems because methylmercury enters the water column in diverse ways then biomagnifies through food webs. At the apex of many freshwater food webs, piscivorous fish can then extend that trophic transfer and potential for neurotoxicity to wildlife and humans. Mining activities, particularly those associated with the San Francisco Bay region, can generate both point and non-point mercury sources. Replicate XANES analyses on largemouth bass and hybrid striped bass from Guadalupe Reservoir (GUA), California and Lahontan Reservoir (LAH), Nevada, were performed to determine predominant chemical species of mercury accumulated by high-trophic-level piscivores that are exposed to elevated mercury in both solution and particulate phases in the water column.
Endovascular stents manufactured from superelastic Nitinol represent a major component in the fight against heart disease. However, accurate characterization of the stress/strain distributions in such stents, which govern their deformation and fracture behavior, is essential for their prolonged safe use in human arteries. Here we report the first direct in situ x-ray micro-diffraction measurements inside the synchrotron of the local strain field (at 10 micron resolution) of a stent-like Nitinol component subjected to realistic multiaxial loading. Our micro-diffraction measurements indicate that state-of-the-art commercial finite-element models are sufficient for predicting local strain fields up to 3%. However, there are significant discrepancies between measured and calculated strains at larger displacements, which result from the continuum-mechanics-based model predictions. Consequently, it is imperative that future development of finite-element models must incorporate effects of transformational strain, phase redistribution, and plastic strain to provide higher fidelity predictions of Nitinol stent performance in vivo.
The mechanics of a basic cellular process found in most living organisms, including humans, is less of a mystery, thanks to work done by Douglas Hattendorf and collaborators, in part at the Stanford Synchrotron Radiation Laboratory (SSRL). The team of researchers, led by Prof. Bill Weis of the Stanford University School of Medicine and of SSRL, solved the structure of a protein that assists in the developmental process of cellular polarization, which gives cells the ability to perform specific biological functions.
Scientists exploring the physics of hearing have found an underlying molecular cause for one form of deafness. The team, led by Gerard Wong, Professor of Materials Science and Engineering, of Physics, and of Bioengineering at the University of Illinois at Urbana-Champaign, report their findings in the February 2007 issue of the journal Physical Review Letters.
Structurally incorporated impurities have been shown to have systematic effects on the rate of the thermally driven transformations in titania nanoparticles. For example, the anatase-to-rutile transformation is slowed when anatase nanoparticles are doped with a cation of valence >+4, but favored when the valence < +4. Based on these observations, Y3+ dopants should promote the anatase-to-rutile transformation. However, prior studies showed that the transformation is actually inhibited by such impurities. So far these [1,2], observations have remained unexplained.
Adaptive immunity relies on the capacity of immune cells to distinguish between the body's own cells and foreign invaders. T-cells are the foot soldiers of the immune system, and they carry receptors that undergo an extensive "education" process for recognizing specific proteins from these invaders. Mature T-cells also show the ability to recognize proteins for which they have not been exposed to. How the T-cell receptors (TCRs) achieve this ability is poorly understood. It is this same immune response which causes T-cell mediated rejection in organ transplant patients, and solving this problem could lead to new ways of combating tissue rejection.
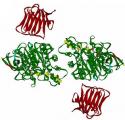
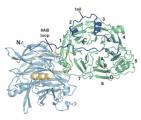
Scientists from Caltech have solved the crystal structure of an ATP-binding Cassette (ABC) transporter called HI1470/1 from the bacteria Haemophilus influenzae. This particular transporter, which is a member of a large family of related proteins prevalent in most organisms including humans, is responsible for moving nutrients across cell membranes. The structure of HI1470/1 exhibits an alternate conformation to that previously observed for the related transporter BtuCD, such that their pathways for moving nutrients open to opposite sides of the membrane. These results give scientists a look at both the beginning and ending stages of how proteins transport nutrients across the membrane bilayers that surround all cells.
The exclusive club of magnetic elements officially has a new member-carbon. Using a proton beam and advanced x-ray techniques, SLAC researchers in collaboration with colleagues from LBNL and the University of Leipzig in Germany have finally put to rest doubts about carbon's ability to be made magnetic.