Mercury toxicity generates environmental concerns in diverse aquatic systems because methylmercury enters the water column in diverse ways then biomagnifies through food webs. At the apex of many freshwater food webs, piscivorous fish can then extend that trophic transfer and potential for neurotoxicity to wildlife and humans. Mining activities, particularly those associated with the San Francisco Bay region, can generate both point and non-point mercury sources. Replicate XANES analyses on largemouth bass and hybrid striped bass from Guadalupe Reservoir (GUA), California and Lahontan Reservoir (LAH), Nevada, were performed to determine predominant chemical species of mercury accumulated by high-trophic-level piscivores that are exposed to elevated mercury in both solution and particulate phases in the water column.
GUA and LAH are both affected either directly or indirectly by the legacy of gold and silver mining in the Sierra Nevada during the nineteenth century. GUA is adjacent to the New Almaden Mercury Quicksilver Mines (NAMQM) in San Jose, CA, the largest elemental-mercury producer in U.S. history. The legacy of those prolific mines unfortunately involves mercury contamination in sediment, water, and biota from cinnabar and calcine deposits ("no consumption" fish advisory since 1987). Total Maximum Daily Load Programs are in development for both the Guadalupe River watershed and down gradient in South San Francisco Bay. LAH is located within the Carson River Mercury Site, the only site in the State of Nevada on the Superfund National Priorities List. Like GUA, LAH's contamination is a mining legacy, but contamination at LAH is derived from elemental mercury imported from mines in the San Francisco Bay area (including NAMQM) and used at mill sites to refine gold and silver ore from the Comstock Lode. Mercury-contaminated mine tailings from the historic mill operations continue to discharge into the Carson River system and accumulate in lentic environments like LAH ("no consumption" fish advisory since 1997).
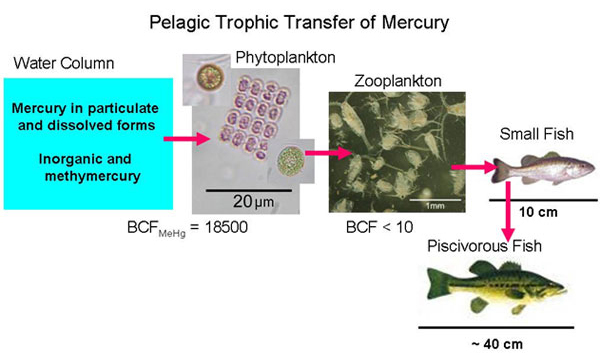
Synchrotron-based X-ray analysis of solid-state mercury speciation serves as a useful complement to traditional analytical methods for mercury by providing detailed mercury speciation (e.g., coordination with protein structures) to more accurately assess risks to human and ecosystem health. As described above, GUA and LAH have distinctly different mercury sources, but both are mining-impacted, mineralogically based, proximal sources in freshwater. In contrast, an extensive survey of fish throughout the Western United States suggested the importance of atmospheric sources to most freshwater systems. Although it is unclear if atmospheric, hydrothermal, or benthic sources of mercury dominate the environment where previously examined marine fish were caught, these marine sources are clearly different from those for GUA and LAH. It is generally assumed that fish accumulate mercury in their tissues predominantly as methylmercury, but notable exceptions have reported significant inorganic mercury in tissues.
Piscivorous fish, all exceeding 300 mm in total length, were collected by angling in GUA, or by electroshocking in LAH in August 2005. The USEPA's human consumption threshold for mercury in fish is 0.3 mg·g-1. Total mercury in our fish samples consistently exceeded that threshold and ranged from 1.74 to 8.28 mg·g-1. The mean (4.05 mg·g-1) was an order of magnitude greater than the mean mercury concentration reported for piscivores of the Western United States (0.26 mg·g-1). In these mining-affected watersheds, extraordinary concentrations of mercury were common even for lower-trophic level organisms such as zooplankton and planktivorous fish (Kuwabara et al., 2005). Bioaccumulation factors (BAF; the ratio of methylmercury concentration in the fish tissue (mg·kg-1) over the dissolved methylmercury concentration in the water (mg·L-1) revealed log BAF values between 7.3 and 8.0, higher than reported for other Hg-contaminated aquatic systems (6.0 and 6.8). These higher BAFs suggested efficient methylmercury trophic transfer at both GUA and LAH.
Hg LIII XANES spectra from our piscivores were compared to ten model compounds. While spectral features of CH3HgS(Cys) showed a striking resemblance to our fresh fish samples at pre- and post-edge regions, features of other model compounds were clearly dissimilar to our fish samples. Regardless of bass species or sampling locations, the predominant mercury species in their muscle tissues was CH3HgS(Cys), containing linear two-coordinate Hg with methyl and cysteinyl sulfur donors. Our findings were consistent with previous work on commercial marine species (Harris et al., 2003) which reported the presence of CH3HgS(Cys).
XANES results demonstrated that mercury was accumulated almost exclusively as methylmercury-cysteine complexes in the muscle tissues of piscivorous freshwater fish from two mining-impacted reservoirs. This result, consistent with observations for several marketed marine fish species, suggested that speciation of bioaccumulated mercury at high trophic levels was consistent over a wide range of ionic strengths and mercury sources. The dominance of methylmercury cysteine complexes in muscle tissues of fish obtained from such contrasting environments and exposure conditions suggested that a generic toxicological model for the consumption of fish could be applicable over a wide range of ecologic settings. Chloro-complexes of methylmercury have long been used as the mercury species of toxicological concern for trophic transfer. This work highlights the importance of describing processes that intra- or extracellularly transform mercury coordination between toxicologically labile and inert mercury complexes.
Kuwabara, J.S.; Topping, B.R.; Moon, G.E.; Husby, P.; Lincoff, A.; Carter, J.L.; Croteau, M.N. (2005) Mercury accumulation by lower trophic-level organisms in lentic systems within the Guadalupe River watershed, California; USGS Scientific Investigations Report 2005-5037 (http://pubs.usgs.gov/sir/2005/5037).
Harris, H.H.; Pickering, I.J.; George, G.N. (2003).The chemical form of mercury in fish. Science 301, 1203.
Kuwabara, J.S., Arai, Yuji, Topping, B.R., Pickering, I. J., and George, G.N. (2007). Mercury speciation in piscivorous fish from mining-impacted reservoirs: Environmental Science and Technology 41, 2745-2749.




