ATP-binding Cassette (ABC) transporters represent a large family of integral membrane proteins, which are found in all organisms from mammals to bacteria. These proteins transport substrates across a biological membrane powered by the energy of adenosine triphosphate (ATP) hydrolysis. ABC transporters primarily consist of two transmembrane domains (TMDs) and two nucleotide binding domains (NBDs) located in the cytoplasm. While diverse with respect to physiological function and TMD architecture, the highly conserved NBDs contain critical sequence motifs for ATP binding and hydrolysis that suggest a common mechanism by which these transporters translocate the substrate across the membrane (1).
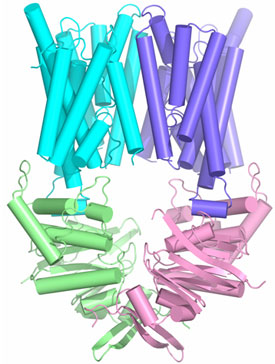
The HI1470/1 transporter from Haemophilus influenzae belongs to the family of binding protein dependent bacterial ABC transporters that mediate the uptake of metal-chelate species including heme and vitamin B12. Using x-ray diffraction data collected at SSRL beam line 9-2, we have determined the x-ray crystal structure of the intact, nucleotide-free HI1470/1 transporter by isomorphous and multi-wavelength anomalous diffraction methods at 2.4 Å resolution (Figure 1). Although the overall architecture of the intact HI1470/1 transporter resembles that of BtuCD (vitamin B12 uptake transporter), a comparison of the x-ray structures reveal differences in tertiary and quaternary arrangements that may be functionally relevant (2). Each of the membrane spanning subunits HI1471 and BtuC contains a total of ten transmembrane helices that are organized into two sets. The TMDs maintain a tapered pathway across the membrane spanning region. Relative to a structurally conserved core composed of seven of these helices, a twist of ~9° about an axis perpendicular to the translocation pathway is required to interconvert the TMDs of the two transporters. Together with the changes in the three remaining helices, these conformational rearrangements result in a translocation pathway that is closed to the periplasm and open to the cytoplasm in HI1470/1, while the converse is observed in BtuCD. The pathways open to opposite sides of the membrane such that HI1470/1 and BtuCD adopt inward and outward facing conformations, respectively (Figure 2).
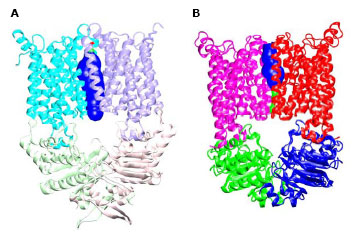
The differences observed between the structures of the HI1470/1 and BtuCD ABC transporters involve relatively modest rearrangements and may serve as structural models for inward and outward facing conformations relevant to the alternating access mechanism of substrate translocation. The roles of binding protein, ligand and particularly nucleotide binding and hydrolysis in driving these conformational transitions remain crucial mechanistic issues that we are presently exploring.
- I. B. Holland, S. P. C. Cole, K. Kuchler, C. F. Higgins, ABC proteins: From Bacteria to Man (Academic, London, 2003).
- K. P. Locher, A. T. Lee, D. C. Rees, Science 296, 1091 (2002).
- O. S. Smart, J. G. Neduvelil, X. Wang, B. A. Wallace, M. S. Sansom, J. Mol. Graph. 14, 354 (1996).
H. W. Pinkett, A. T. Lee, P. Lum, K. P. Locher, D. C. Rees. (2007) An Inward-Facing Conformation of a Putative Metal-Chelate-Type ABC Transporter. Science 315, 373 - 377.

