The cells that comprise many tissues are polarized, meaning that they have distinct 'sides' with different membrane identities. For example, in the cells that line the intestine, the membrane that faces the space in the gut has special proteins responsible for uptake of nutrients, whereas the sides that contact neighboring cells have different proteins on their surfaces. Cell polarity is fundamental to many aspects of cell and developmental biology and it is implicated in differentiation, proliferation and morphogenesis in both unicellular and multi-cellular organisms. Loss of cell polarity can lead to uncontrolled tissue growth and cancers. To generate and maintain this polarized structure, specific proteins and lipids must be delivered to particular locations on the cell membrane. This process involves active transport of membrane-enclosed vesicles containing specific cargo to a target site, where the vesicle and target membranes then fuse to deliver the cargo. Soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins in the vesicle membrane and target membrane form a complex that mediates membrane fusion. The process by which SNARE-mediated membrane fusion is coordinated with the machinery that transports the vesicle to the correct location is poorly understood.
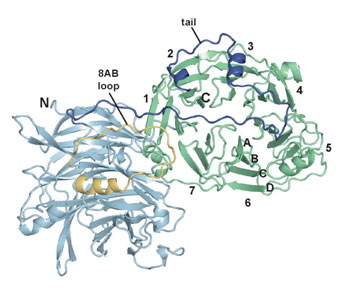
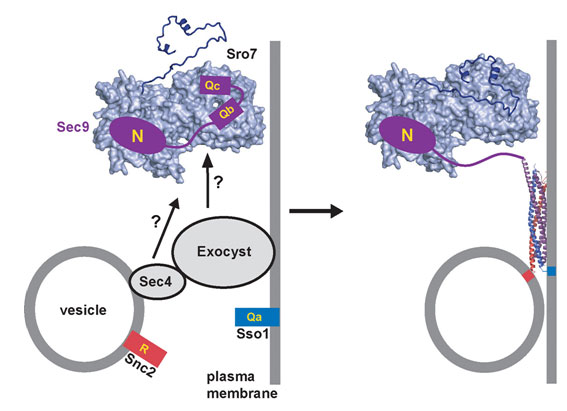
In this work, Hattendorf et al. studied a yeast protein called Sro7 that is essential for delivery of vesicles from a mother to a budding daughter cell, which is another example of polarized cell growth. Sro7 and its relatives in higher organisms bind to SNARE proteins and are known to be essential for cell polarity, but their mechanism is unknown. The crystal structure of Sro7, determined with data measured at SSRL (Beam Line 11-1) and the Advanced Light Source, revealed a double-domain structure that is followed by a "tail" that binds to the surface of one of the domains (Figure 1). It was shown that removal of the tail promotes binding to a yeast SNARE protein and thereby blocks formation of SNARE complexes. Thus, the structure led to the discovery of an unanticipated mechanism for regulating SNARE complex assembly (Figure 2), and provided the first mechanistic data on this essential family of proteins.
This work was supported by grants from the NIH and the American Cancer Society.
- Pruyne, D., Legesse-Miller, A., Gao, L., Dong, Y. & Bretscher, A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol 20, 559-91 (2004).
- Jahn, R., Lang, T. & Südhof, T.C. Membrane fusion. Cell 112, 519-533 (2003).
D. A. Hattendorf, A. Andreeva, A. Gangar, P. J. Brennwald, and W. I. Weis. (2007). Structure of the yeast polarity protein Sro7 reveals a SNARE regulatory mechanism. Nature 446, 567-571.




