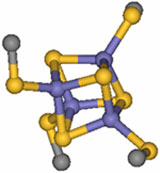
Proteins containing Fe4S4 iron-sulfur clusters are ubiquitous in nature and catalyze one-electron transfer processes. These proteins have evolved into two classes that have large differences in their electrochemical potentials: high potential iron-sulfur proteins (HiPIPs) and bacterial ferredoxins (Fds). The role of the surrounding protein environment in tuning the redox potential of these iron sulfur clusters has been a persistent puzzle in biological electron transfer [1]. Although HiPIPs and Fds have the same iron sulfur structural motif - a cubane-type structure - (Figure 1), there are large differences in their electrochemical potentials. HiPIPs react oxidatively at physiological potentials, while Fds are reduced. Recently, sulfur K-edge x-ray absorption spectroscopy (XAS; measured at SSRL beam line 6-2) has been used to uncover the substantial influence of hydration on this variation in reactivity in a collaborative effort led by Stanford Chemistry and Photon Science researchers Edward I. Solomon, Keith O. Hodgson and Britt Hedman [2].
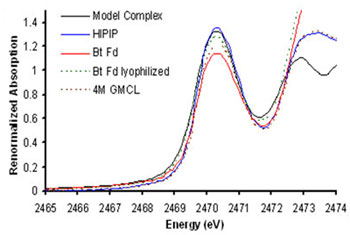
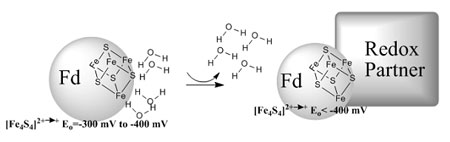
The sulfur-K XAS pre-edge involves excitation of S 1s electrons to the unoccupied valence orbitals formed by interaction of the Fe 3d orbitals with the S 3p orbitals [3]. Since the transition is localized on the sulfur, the pre-edge provides a direct measure of S 3p character in the metal d orbitals (i.e. covalency). In this study, which follows several years of developing the methodology behind this approach [4], S K-edge XAS was used to examine the changes in covalency in native and perturbed (lyophilized Fd and unfolded HiPIP) protein environments. These experiments show that the Fe-S covalency is much lower in natively hydrated Fd active sites than in HiPIPs, but increases upon water removal (Figure 2). Similarly, HiPIP covalency decreases upon unfolding, exposing an otherwise hydrophobically shielded active site to water (Figure 3). These results demonstrate that Fe-S covalency is a direct experimental marker of the local electrostatics due to H-bonding. Studies on related model compounds and accompanying density functional theory (DFT) calculations support a correlation between Fe-S covalency and ease of oxidation, which suggests that differential hydration accounts for most of the difference between Fd and HiPIP reduction potentials. This raises the intriguing possibility that oxidation/reduction potentials can be regulated by protein/protein and protein/DNA interactions that effect cluster hydration.
- K. Fukuyama, Handbook of Metalloproteins; A. Messerschmidt, R. Huber, T.L. Poulos, K. Wieghardt, Eds, John Wiley & Sons, Ltd, pp. 543 (2001).
- A. Dey, F.E. Jenney Jr., M.W.W. Adams, E. Babini, Y. Takahashi, K. Fukuyama, K.O. Hodgson, B. Hedman, E.I. Solomon, Science 318, 1464 (2007).
- T. Glaser, B. Hedman, K.O. Hodgson, E.I. Solomon, Acc. Chem. Res. 33, 859 (2000).
- B. Hedman, K.O. Hodgson, E.I. Solomon, J. Am. Chem. Soc. 112, 1643 (1990); E.I. Solomon, B. Hedman, K.O. Hodgson, A. Dey, R.K. Szilagyi, Coord. Chem. Rev., 249, 97 (2005).
A. Dey, F. E. Jenney, Jr., M. W. W. Adams, E. Babini, Y. Takahashi, K. Fukuyama, K. O. Hodgson, B. Hedman and E. I. Solomon, "Solvent Tuning of Electrochemical Potentials in the Active Sites of HiPIP Versus Ferredoxin", Science 318, 1464 (2007)

