Proteins are delivered to specific sites within cells in small membrane-enclosed carriers called transport vesicles. Transport vesicles are targeted to the correct acceptor membrane by a number of sequential steps that are regulated by small GTPases of the Rab and Arf families. Small GTP-binding proteins (GTPases) are a large group of proteins involved in the regulation of quite different cellular processes like cell proliferation, differentiation (Ras-, Rap- and Ral-family), nuclear transport (Ran), vesicular transport (Rab-family) and cytoskeleton organization (Rho- and Arf-family). Vesicles are transported along microtubule or actin tracks; target recognition is thought to involve a molecular "tethering" event at the target membrane that is mediated by coiled-coil and multi-subunit tethers, prior to membrane fusion.
GCC185 is a large (185 kDa) coiled-coil protein at the Golgi complex believed to mediate the initial interaction between incoming vesicles and the Golgi membrane, functioning as a tethering factor. In addition, GCC185 was recently shown to have a second function in recruitment of proteins that catalyze microtubule polymerization from the Golgi.
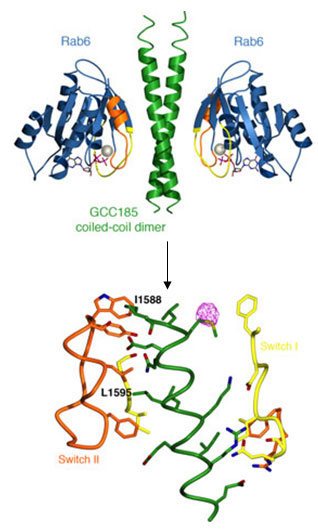
Given that GCC185 plays vital roles both in organizing the cell cytoskeleton and in vesicle traffic, Schweizer Burguete et al. investigated how the putative tether itself is localized to the Golgi membrane. We have shown that Golgi-recruitment of GCC185 is mediated by the cooperation of two Golgi-localized small GTPases belonging to the Rab and Arf families. Rab6 binding to GCC185 promotes the subsequent binding of Arl1 to an immediately adjacent domain. Biochemical analysis of these interactions revealed a helical, dimeric, Rab6 binding domain in GCC185. The crystal structure of a complex between the Rab binding domain (RBD) of GCC185 and Rab6 was determined using diffraction data obtained at SSRL (Beam Lines 11-1 and 7-1) and provided the stoichiometry and the molecular details of this interaction. Rab6 switch I and II regions, which adopt a specific conformation when the protein is GTP-bound, contact a dimeric coiled-coil in GCC185 with two-fold symmetry, and residues critical for Rab binding and Golgi-localization of GCC185 lie in the binding interface (Figure 1).
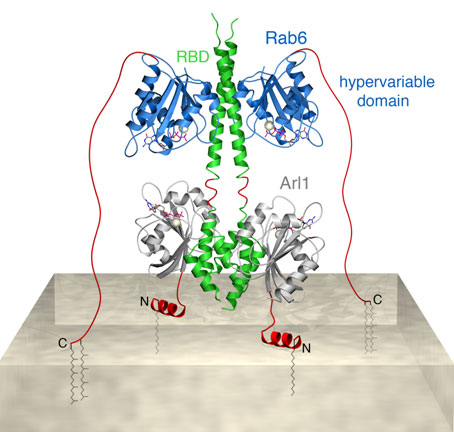
Based on our observations we have created a structure-derived model for simultaneous GTPase binding to the carboxy-terminal region of GCC185. In this model the three proteins form a hetero-hexameric complex that attach the 185 kDa tether to the surface of Golgi membranes (Figure 2). This model highlights how Arf and Rab-family members may interact with the same binding partner at different distances from the membrane. The Rab GTPases are expected to reach binding sites as far as 10 nm away from the membrane via their unstructured and membrane-anchored, C-terminal tails. Arf GTPases on the other hand, will bind to membrane-proximal domains, enabling cooperation with Rab proteins in determining the fate of a common binding partner.
Reddy, J. V., Burguete, A. S., Sridevi, K., Ganley, I. G., Nottingham, R. M. and Pfeffer, S. R. (2006). A functional role for the GCC185 Golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 17(10):4353-4363.
Efimov, A., Kharitonov, A., Efimova, N., Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N., Maia, A. R., McLeod, I. X., Yates, J. R., Maiato, H., Khodjakov, A., Akhmanova, A. and Kaverina, I. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 12(6):917-30.
Panic, B., Perisic, O., Veprintsev, D. B., Williams, R. L. and Munro, S. (2003) Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell. 12(4):863-74.
Schweizer Burguete, A., Fenn T. D., Brunger, A. T. and Pfeffer, S. R. (2008). Rab and Arl GTPase Family Members Cooperate in the Localization of the Golgin GCC185. Cell 132(2), 286-298.




