The rise in obesity in the United States parallels a dramatic increase in obesity-associated diseases, most notably type-2 diabetes. This disease is predicted to reach epidemic proportions in the next several decades (Zimmet et al 2001, Urek et al 2007). Thus, understanding the biochemical processes underlying type-2 diabetes and identifying new targets for therapeutic intervention are critical for national and world health. A drug of choice to treat type-II diabetes is pioglitazone, a thiazolidinedione (TZD) derivative originally thought to exert its effect through activation of the nuclear transcription factor PPARg. Recently, a novel protein target for pioglitazone was discovered and was called mitoNEET (Colca et al 2004). This protein is anchored to the outer mitochondrial membrane (OMM) (Wiley et al 2007). Contrary to predictions that this was a zinc-finger transcription factor we discovered that mitoNEET is a novel 2Fe-2S protein.
In an effort to understand the structural properties of this protein, a soluble form of recombinant human mitoNEET was crystallized in an orthorhombic space group P212121, with unit-cell parameters a = 46.81 Å, b = 49.62 Å, c = 59.01 Å (Paddock et al 2007). The structure was determined by x-ray diffraction from 1.5 Å resolution data collected from SSRL Beamline 9-2. Initial phasing was obtained from Fe-MAD (multiwavelength anomalous dispersion) datasets collected at wavelengths corresponding to the inflection, high energy remote, and absorption peak of Fe. Data was processed using automated MAD script developed at SSRL. The structural model was refined to an R-factor = 18.2 %.
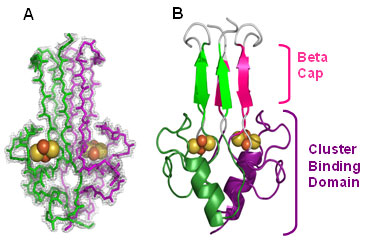
The crystal structure reveals that mitoNEET folds into a unique homodimeric structure with one 2Fe-2S cluster bound to each monomer within the dimmer (Figs. 1 & 2). A structural similarity search revealed that this fold is novel when compared with the >650 known Fe-S proteins, and it is also unique when compared with the >44,200 known members of the structural databases. The protein is folded into two spatially distinct subregions: a b-rich or "b-cap" domain and a helical 2Fe-2S binding or "cluster-binding" domain (Fig. 1B). The b-rich domain contains a strand swap from opposite ends of the primary sequence to form the b-cap structure (Fig. 1B).
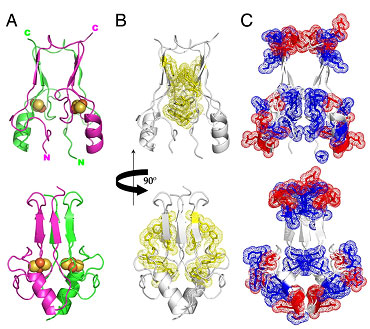
A prominent feature of the structure is the presence of two 2Fe-2S clusters that are separated by ≈ 16 Å from each other within the larger helical cluster-binding domain (≈ 30 Å across) (Fig. 2). The mitoNEET dimer has an unusual distribution of aromatics (Fig. 2B) forming a ring around the central b-sheet region and charges (Fig. 2C) that create an internal macro-dipole. Three Cys residues and one histidine residue on each monomer are ligands to the 2Fe-2S clusters. Interestingly, mitoNEET shares this unusual 3Cys cluster coordination with the structurally unrelated cluster scaffold protein IscU (Ramelot et al 2004).
Complementary biophysical investigations showed that the cluster is redox active and labile below pH 8.0 (Wiley et al 2007), characteristics possibly related to its function. Optical and NMR experiments demonstrated that the presence of the diabetes drug pioglitazone increased the stability by ≈ 10-fold. The unusual lability was associated with the coordinating ligand His-87 (Wiley et al 2007), which cannot serve as a stabilizing ligand for the 2Fe-2S when protonated. This unusual characteristic of the protein raises the interesting possibility that mitoNEET participates in Fe-S cluster assembly or storage. We predict that the cluster-binding domain is situated near the OMM in vivo placing mitoNEET in a unique position to receive and transfer clusters that have crossed the outer mitochondrial membrane, a process that is not presently fully understood.
Although TZDs activate peroxisome proliferator-activating receptors (PPARg), data suggesting alternative modes of action involving mitochondria has accumulated. Our structural results may have important implications for both mechanisms of drug action and future optimization of TZDs, especially in light of the fact that the structures of PPARg and mitoNEET are nearly completely dissimilar. Whether the beneficial effects of TZDs on mitochondria including biogenesis and normalization of lipid oxidation are mediated through mitoNEET is unknown. However, these data, combined with those of Colca et al. (1994), suggest that pioglitazone can bind and alter the properties of mitoNEET that is expressed in many insulin-responsive tissues. Although further biological and biophysical experiments are needed to relate in vitro binding to in vivo effects, mitoNEET may prove to be an alternative target for drug actions.
This work was supported by that National Institutes of Health (GM 41637, GM18024, GM 18849, GM54038, and DK54441) and the Zevi Hermann Shipira Foundation. Portions of this research were carried out at the SSRL, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.
Colca, J. R., McDonald, W. G., Waldon, D. J., Leone, J. W., Lull, J. M., Bannow, C. A., Lund, E. T. & Mathews, W. R. (2004) Am J Physiol Endocrinol Metab 286, E252-260.
Ramelot, T.A., Cort, J. R., Goldsmith-Fischman, S., Kornhaber, G. J., Xiao, R., Shastry, R., Acton, T. B., Honig, B., Montelione, G. T. & Kennedy, M. A. (2004) J Mol Biol 344, 567-583.
Urek, R., Crncevic-Urek, M. & Cubrilo-Turek, M. (2007) Acta Med Croatica 61, 161-164.
Wiley, S. E., Paddock, M. L., Abresch, E. C., Gross, L., van der Geer, P., Nechushtai, R., Murphy, A. N., Jennings, P. A. & Dixon, J. E. (2007) J Biol Chem. 282, 23745 - 23749.
Zimmet, P., Alberti, K. G. and Shaw, J. (2001) Nature 414, 782-787.
Paddock, M. L., Wiley, S. E., Axelrod, H. L., Cohen, A. E., Roy, M., Abresch, E. C., Capraro, D., Murphy, A. N., Nechushtai, R., Dixon, J. E. and Jennings, P. A. (2007) MitoNEET is a uniquely folded 2Fe-2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci USA 104, 14342-14347.




