The 2022 mpox outbreak highlighted the serious threat posed by the mpox virus (MPXV) with unprecedented geographic spread and sustained human-to-human transmission, alongside mutations that raise concerns about the virus becoming more transmissible and resistant to current vaccines and treatments.[1,2] Current therapeutic options remain limited and inadequate for controlling severe infections. While the MVA-BN (JYNNEOS) vaccine can reduce disease severity when administered up to 4 days post-exposure, it is insufficient to fully prevent infection and induces only modest, short-lived antibody responses.[3,4] Existing antiviral drugs including Tecovirimat and Brincidofovir have shown insufficient efficacy in clinical trials, with Tecovirimat failing to demonstrate significant benefit.[5] These therapeutic shortcomings are particularly problematic for immunocompromised individuals and those at high risk for severe disease, creating an urgent need for more effective, targeted interventions that can provide robust protection against mpox and potentially other emerging orthopoxviruses.
In a large consortium, researchers identified and structurally characterized three human monoclonal antibodies (EV35-2, EV35-6, and EV35-7) that provide potent protection against mpox virus through targeting the A35 envelope protein, a critical component of the virion essential for cell-to-cell viral spread.[6] The antibodies were isolated from a mpox-convalescent individual and showed high-affinity binding to both MPXV A35 and vaccina virus (VACV) A33 proteins, with low dissociation constants in the pico- to nano-molar range.
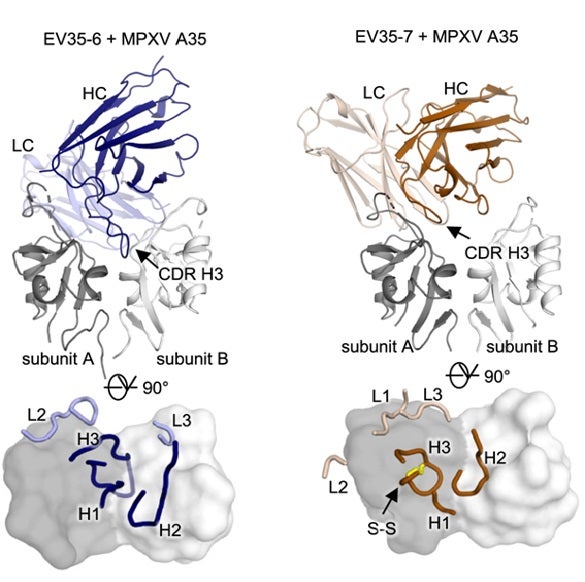
Structural analysis revealed that all three antibodies bind to a highly conserved epitope located within the groove formed by the MPXV A35 dimer, as shown in the co-crystal structures depicted in Fig. 1. The complexes were determined at high to moderate resolution (2.2 - 3.3 Å) using X-ray crystallographic data collected at SSRL BL12-1 and at NSLS-II. These structures demonstrate that EV35-2 and EV35-7 both employ a conserved sequence motif in their main binding regions that serves as an anchor point for binding to the same target site on the viral protein, while EV35-6 employs a distinct but overlapping binding mode with a long binding loop (CDR H3) that extends into a unique hydrophobic pocket. The structures show that each antibody makes extensive contact with the viral protein, binding to both parts of the A35 protein complex through several key interaction sites.
In vivo protection studies showed that all three antibodies provided complete prophylactic protection against lethal VACV infection, while against MPXV challenge, EV35-6 and EV35-7 achieved 100% protection compared to 75% for EV35-2. EV35-6 also demonstrated therapeutic efficacy, providing complete protection when administered 24 hours after initial infection. The antibodies function through both direct viral neutralization and immune system recruitment, with EV35-2 showing greater dependence on recruitment, Importantly, analysis of human sera from mpox-convalescent individuals revealed that higher levels of antibodies competing with these three antibodies correlated with improved clinical outcomes, including shorter symptom duration and reduced hospitalization rates. The structural and functional results establish A35 as a critical therapeutic target and highlight these antibodies as promising candidates for next-generation mpox treatments.
Primary Citation
R. F. Fantin, M. Yuan, S.-C. Park, B. Bozarth, H. Cohn, M. Ignacio, P. Earl, A. Civljak, G. Laghlali, D. Zhang, X. Zhu, J. Crandell, V. Monteiro, J. J. Clark, C. Cotter, M. Burkhardt, G. Singh, P. Warang, J. García-Bernalt Diego, K. Srivastava, L. A. Lugo, L. Pischel, I. Yildirim, S. B. Omer, D. da Silva, F. Krammer, G. Bajic, V. Simon, M. Schotsaert, C. Lucas, I. A. Wilson, B. Moss and C. H. Coelho, "Human Monoclonal Antibodies Targeting A35 Protect from Death Caused by mpox", Cell 188, (2025) doi: https://doi.org/10.1016/j.cell.2025.08.004
References
- Chen, Y., Li, M., and Fan, H. The monkeypox outbreak in 2022: adaptive evolution associated with APOBEC3 may account for. Signal Transduct. Target. Ther. 7, 323 (2022). https://doi.org/10.1038/s41392-022-01181-x
- O'Toole, Á., Neher, R.A., Ndodo, N., Borges, V., Gannon, B., Gomes, J.P., Groves, N., King, D.J., Maloney, D., Lemey, P., Lewandowski, K., Loman, N., Myers, R., Omah, I.F., Suchard, M.A., Worobey, M., Chand, M., Ihekweazu, C., Ulaeto, D., Adetifa, I., and Rambaut, A. APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science 382, 595 (2023). https://doi.org/10.1126/science.adg8116
- Oom, A.L., Wilson, K.K., Yonatan, M., Rettig, S., Youn, H.A., Tuen, M., Shah, Y., DuMont, A.L., Belli, H.M., Zucker, J.R., Rosen, J.B., Herati, R.S., Samanovic, M.I., Duerr, R., Kottkamp, A.C., Mulligan, M.J., and the NYC OSMI Study Group. The two-dose MVA-BN mpox vaccine induces a nondurable and low avidity MPXV-specific antibody response. J. Virol. 99, e0025325 (2025). https://doi.org/10.1128/jvi.00253-25
- Collier, A.Y., McMahan, K., Jacob-Dolan, C., Liu, J., Borducchi, E.N., Moss, B., and Barouch, D.H. Rapid Decline of Mpox Antibody Responses Following MVA-BN Vaccination. Preprint at medRxiv (2024). https://doi.org/10.1101/2024.09.10.24313399
- National Institutes of Health. Topline Results from PALM 007 Study of SIGA's Tecovirimat in Treatment of Mpox Released (SIGA Technologies) (2024). Available at: https://investor.siga.com/investors/news/news-details/2024/Topline-Results-from-PALM-007-Study-of-SIGAs-Tecovirimat-in-Treatment-of-Mpox-Released/
- Blasco, R., and Moss, B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66, 4170 (1992).https://doi.org/10.1128/JVI.66.7.4170-4179.1992