Anthropologists learn about ancient cultures through the objects left behind. Ritualistic artifacts give glimpses into the religious and belief systems of nonextant societies. Application of new techniques of chemical and structural analysis to the study of ancient objects can give more insight into how they were made and used.
Approximately 1,700 scientists visit SSRL annually to conduct experiments in broad disciplines including life sciences, materials, environmental science, and accelerator physics. Science highlights featured here and in our monthly newsletter, Headlines, increase the visibility of user science as well as the important contribution of SSRL in facilitating basic and applied scientific research. Many of these scientific highlights have been included in reports to funding agencies and have been picked up by other media. Users are strongly encouraged to contact us when exciting results are about to be published. We can work with users and the SLAC Office of Communication to develop the story and to communicate user research findings to a much broader audience. Visit SSRL Publications for a list of the hundreds of SSRL-related scientific papers published annually. Contact us to add your most recent publications to this collection.
The capsid that surrounds viruses is formed from subunit proteins that interact in specific ways to form a tight shell. The processes of coming together and forming interactions are multistep and complex and are fundamental events to acquire viral infectivity. The capsid maturation process of the Nudaurelia capensis omega virus includes pH-dependant conformational changes and auto-proteolysis. Like many human viruses such as HIV and herpes virus, NwV, an insect virus, requires these specific structural changes to become infectious.
The 3D structure of bone is critical for maintaining strength. Skeletal diseases such as osteoporosis and environmental conditions such as weightlessness, radiation, and vitamin D deficiency can affect bone structure. Understanding the 3D structure of bone is critical to understanding how these conditions affect bone's form and function.
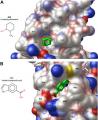
HIV protease is a common and critical drug target for combating HIV infection and AIDS. As HIV develops resistance to anti-viral drugs, new therapies are required. Since most of the virus's mutations that confer drug resistance cluster in the active site of the protease, scientists are interested in molecules that may bind other places on the enzyme. Computer simulations aid the design of drugs and fragments, which are smaller than typical drugs, to bind the enzyme's surface in a way that compliments the activity of traditional active-site binding drugs.
Archaeopteryx, the half-reptilian, half-avian creature that lived 150 million years ago is famous as the fossil record's link between dinosaurs and birds. The discovery of the firstArchaeopteryx fossil, which coincided with the publication of Charles Darwin's On the Origin of Species, provided strong evidence of the theory of evolution. Because Archaeopteryxfossils are important and rare, no samples have been taken for standard chemical analysis, which is a destructive process.
An unusual property of the last year's H1N1 "swine flu" virus pandemic is that it disproportionately affected the young. People over the age of around 65 showed much less vulnerability than to more typical flu strains, suggesting that they might have been exposed to a similar virus over three decades ago. Another atypical property of the 2009 H1N1 strain is that its hemagglutinin (HA) subunit is the same subtype as the regular seasonal strains, whereas most pandemics are caused by viruses with novel HA domains.
Cells need copper to function, but too much copper can be toxic, leading to liver damage and neurological problems, as happens in disorders such as Wilson disease. The inorganic small molecule tetrathiomolybdate (TM), assumed to be a copper chelator, is commonly used to treat Wilson disease. TM may also be an effective treatment of some cancers by starving the cancer cells of the copper they need to grow. Despite its common use, its molecular mechanism was unknown.
The potential for using biological enzymes to make hydrogen to use as a renewable energy source is a hot topic, but little is known about how these complex enzymes assemble and work. The [FeFe]-hydrogenase enzyme binds iron and sulfur ions to catalyze the reversible production of hydrogen ions from protons and electrons. The enzyme's active site, termed the H-cluster, uses a complex Fe-S cluster comprised of a [4Fe-4S] subcluster and a 2Fe subcluster to catalyze the reaction.
Superconductivity is a hot topic in physics for good reason. With an electrical resistance of zero, superconductors transport electrical current with no loss of energy. Unfortunately, scientists have only found materials to be superconducting at very low temperatures, much too low for widespread use. In the 1980s, scientists discovered a class of "high-temperature" superconductors that can be used at the temperature of liquid nitrogen (~-200°C).
Hydrogen fuel cells are a green alternative to fossil fuels for powering vehicles, since the byproduct of fuel cell energy production is simply water. A problem with using fuel cells is their high cost, largely due to the use of the expensive element platinum in their design. The platinum is used at the cathode of the fuel cell, where it catalyzes the reduction of oxygen molecules into oxygen atoms. It is a good choice for this role because it binds the reactant well, breaks the O-O bond efficiently, and does not bind too tightly to the products.






![Figure. 1: Structure of the nest-shaped [S6Cu4MoS4] cluster in the [TM][(Cu)(Cu-Atx1)3] trimer complex. (A) Structure of the [S6Cu4MoS4] cluster with average interatomic distances. (B) Cu anomalous peaks in the final model of the [S6Cu4MoS4] cluster (blue mesh of the anomalous difference Fourier map are contoured at 10.0 s level) Figure 1.](https://www-ssrl.slac.stanford.edu/content/sites/default/files/styles/custom-160/public/images/science/highlights/2010/tm-molmech_fig1.jpg?itok=6qIgQaz3)
![Figure 1. Top: overall ribbon and surface representation of the x-ray crystal structure of [FeFe]-hydrogenase HydAdeltaEFG. Loop regions important for the cluster insertion process are colored green. Bottom: Ball and stick representation of the active site of HydAdeltaEFG at which a [4Fe-4S] cluster is present. Coloring scheme: rust, iron; orange, sulfur; gray, carbon; red, oxygen; blue, nitrogen. Figure 1.](https://www-ssrl.slac.stanford.edu/content/sites/default/files/styles/custom-160/public/images/science/highlights/2010/fe-s_clusters_fig1.jpg?itok=-AqeFqIi)





