Scientists studying osteoporosis and other skeletal diseases are interested in the 3D structure of bone and its responses to conditions such as weightlessness, radiation (of particular interest to astronauts) and vitamin D deficiency. The current gold standard, micro-computed tomography (micro-CT), provides 3D images of trabeculae, the small interior struts of bone tissue, and electron microscopy can provide nanometer resolution of thin tissue slices. Hard X-ray transmission microscopy has provided the first 3D view of bone structure within individual trabeculae on the nanoscale.
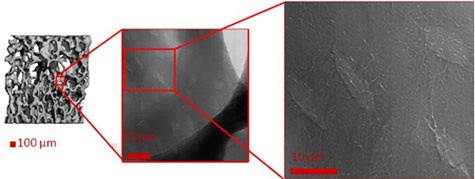
Bone material responds to mechanical stresses such as experienced during load-bearing, or its opposite weightlessness, to form new bone or break down existing bone by complex signals. These signals are believed to be sent and received from bone cells (osteocytes) housed within mineralized cavities called lacunae, via cell processes that pass nutrients and other signals within mineralized channels (canaliculi) extending from the lacunae (Duncan & Turner 1995, Khosla et al. 2008). Therefore, obtaining the 3D structure of lacunae and canaliculi at the nanoscale will help our understanding of healthy bone tissue and the changes that occur with aging and disease.
A team that included scientists from NASA Ames Research Center, Cornell University, Xradia, Inc. and the Stanford Synchrotron Radiation Lightsource (SSRL) has conducted imaging experiments of bone using the transmission x-ray microscope (TXM) on SSRL beam line 6-2. They imaged trabeculae from mice that were hindlimb unloaded, a method developed by NASA to simulate weightlessness, reducing the functional loads experienced by the hindlimbs. 2D images show detailed structures of lacunae and the associated canaliculi (Figure 1), and tomography provided a 3D view of these structures (Figure 2, and supplemental movie). Tomography was obtained by rotating the bony trabecula and taking images at successive angles from -90 to 90 degrees. These images were reconstructed mathematically to provide a 3D image, and 2D slices from the 3D data (Fig. 2 b and c) provide detail of the lacuna and canaliculi extending from the cell cavity.
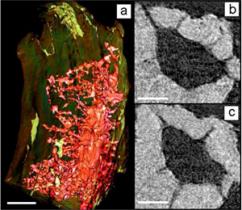
Trabecular bone density was also measured quantitatively in this study, by first calibrating TXM absorption by apatite, which is the main component of mineralized phase in bone that absorbs X-rays. From tomographic slices of the bone, tissue absorption was compared to that of pure apatite, to quantify density differences within trabeculae on the nanoscale. The resolution of this information is substantially better than can be obtained from an averaged microscale bone density as determined by micro-CT. This nano-CT TXM method can determine localized density changes, for example to compare newly formed vs. older tissue after treatments to simulate and ameliorate bone disease.
The ability to image networks of osteocyte lacunae and canaliculi and to measure local bone tissue density changes at high resolution will have significant impact on our understanding of skeletal adaptation and disease. This approach is already providing a novel understanding of the nanostructure and properties of complex mineralized biological specimens and holds the promise of being greatly informative about the nanostructure of materials and the nanoscale complexity of life.
- Duncan, R.L. & Turner, C.H. 1995. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344-358.
- Khosla, S., Westendorf, J.J. & Oursler, M.J. 2008. Building bone to reverse osteoporosis and repair fractures. J Clin Invest 118: 421-428.
Andrews, J.C., Almeida, E., van der Meulen, M.C.H., Alwood, J.S., Lee, C., Liu, Y., Chen, J., Meirer, F., Feser, M., Gelb, J., Rudati, J., Tkachuk, A., Yun, W., Pianetta, P. 2010. Nanoscale X-ray Microscopic Imaging of Mammalian Mineralized Tissue. Microscopy and Microanalysis 16(3): 327-336.




