Magnetic data storage devices are ubiquitous in our modern, data-rich world. Computer hard disks, magnetic recording tape, and magnetic strips on credit cards use such devices, creating pressure to engineer ever greater data density on smaller surfaces.
Approximately 1,700 scientists visit SSRL annually to conduct experiments in broad disciplines including life sciences, materials, environmental science, and accelerator physics. Science highlights featured here and in our monthly newsletter, Headlines, increase the visibility of user science as well as the important contribution of SSRL in facilitating basic and applied scientific research. Many of these scientific highlights have been included in reports to funding agencies and have been picked up by other media. Users are strongly encouraged to contact us when exciting results are about to be published. We can work with users and the SLAC Office of Communication to develop the story and to communicate user research findings to a much broader audience. Visit SSRL Publications for a list of the hundreds of SSRL-related scientific papers published annually. Contact us to add your most recent publications to this collection.
The heat that builds up in the shuttling of current in electronics is an important obstacle to packing more computing power into ever-smaller devices: Excess heat can cause them to fail or sap their efficiency.
Angle-resolved photoemission spectroscopy (ARPES) measurements taken at Beam Line 5-4 at SSRL and at the Advanced Light Source have observed an exotic property that could warp the electronic structure of a material in a way that reduces heat buildup and improves performance in ever-smaller computer components.
When a geographical area is contaminated with radioactive elements, time and heat can cause them to combine with other atoms to form a variety of compounds. Knowing what compounds form and when they form is important for containing and cleaning contaminated sites. Computer models can make predictions but are limited to the currently known reactions and compounds that can be described in the laboratory. A collaboration of scientists has taken samples from the fields of six different contaminated sites to discover which chemical species are formed from uranium and plutonium. The sites studied released these elements under different circumstances and into different environments.
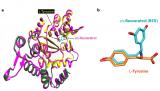
Famous for its presence in red wine, the molecule resveratrol is present in many foods, including grapes, blueberries, and peanuts. Studies showing that resveratrol can elicit health benefits, including longevity in animals, have generated much interest in its effects on humans and its mechanisms of action. These are partly unknown but, recently, scientists found resveratrol can affect a stress response pathway associated with longevity.
Creating novel enzymes to perform specific chemical reactions is a field of great promise, but it is still in its early stages. Efforts usually involve using well-studied protein structural and functional domains to create new active sites. Scientists have recently developed a different approach, creating the active site in the interface between proteins in a multi-protein complex. They started with a well-researched, natural protein that, in its natural state, does not form complexes with other proteins, and nor does it catalyze the desired reaction.
The sun provides more energy than what could ever possibly be consumed. However, switching to solar energy to end our dependence on fossil energy resources is made difficult not merely by how much is consumed, but rather by the pattern of how energy is used: significant amounts are consumed by road and air transportation and must be provided “on board” in the form of fuels. This problem could be solved with new devices that convert sunlight into renewable fuels, for example, by driving a light-induced current between two electrodes that split water by electrolysis into hydrogen and oxygen. Currently, the limiting step for the viability of this process is the oxygen evolution reaction (OER) that takes place at the anode.
One of the most important processes used in petroleum refineries is called fluid catalytic cracking (FCC). This chemical process converts large or heavy molecules of crude oil into smaller and lighter hydrocarbons, such as gasoline. This useful conversion is due in great part to a tiny catalyst particle just 50 to 150 millionths of a meter in diameter. The particle consists of a complex mixture of silica-alumina, clay and zeolite in a porous structure that enables the crude oil molecules to flood the material and reach the catalytically active areas within the particle. After the conversion process, this structure also allows the lighter molecules to leave the catalyst.
As a basic biological building block of amino acids and DNA, nitrogen is necessary for life. Yet most of the Earth’s nitrogen is contained in the atmosphere as dinitrogen, which most organisms are unable to use because they cannot break dinitrogen’s N-N-triple bond. A few microorganisms, however, are able to use an enzyme called nitrogenase to catalyze the transformation of dinitrogen into bioavailable ammonia.
Advanced permanent magnets with low cost and high energy density are important for next-generation technologies, and one promising type of advanced magnet is the exchange spring magnet. This type of nanocomposite comprises two phases of magnetic materials: “hard” magnets, which can remain uniformly magnetized under large fluctuations in magnetic fields, and are often made of rare earth elements, and “soft” magnets, which have a high energy density but their magnetic states can easily be disturbed by small magnetic fields.
Lithium-ion batteries, the mobile power source for most electronic devices, play an important role in everyday life. In the coming decades, they could play an even greater role, powering electric vehicles or storing electrical energy for the grid – if researchers can find ways to improve them.
In particular, the energy density of current batteries is limited by the capacity of the positive electrode, which in turn is determined by the properties and concentration of its active material. By better understanding this material and its limitations, researchers hope to design the highest capacity electrodes possible.