All living organisms depend on the availability of nitrogen for incorporation into the basic biological building blocks such as amino acids and DNA. Globally the largest reservoir for nitrogen is the atmosphere, with an N2 content of roughly 78%. However, as a highly unreactive gas, most organisms are unable to directly utilize dinitrogen due to the severe energy barrier required to break the N-N-triple bond. Only relatively few microorganisms are capable of accomplishing this energy demanding reaction. A common factor for all these “N2-fixing“ systems is that only a single enzyme has apparently emerged that is capable of catalyzing this reduction. The biological process occurs through the activity of nitrogenase, transforming N2 into bioavailable ammonia. Today, roughly 50% of the global N2 reduction is catalyzed by this process, while the industrial Haber-Bosch-process accounts for the other half to provide nitrogen-fertilizer for crop growth. Therefore, the Haber-Bosch process indirectly feeds half of the world's population today. However, the downside of the industrial pathway is the high-energy consumption as well as side effects of fertilization that lead to severe environmental impacts such as a nitrate poisoning of the soils.
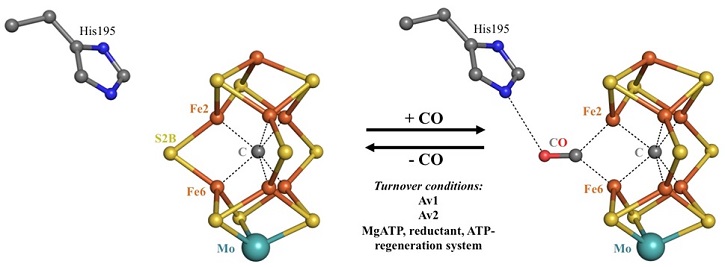
Figure 1. Reversible carbon monoxide binding to the FeMo-cofactor. Upon protein turnover in the presence of CO, one of the belt-sulfur atoms of the metal center is reversible replaced by the N2 isoelectronic ligand. CO binds in a μ2-bridging mode to Fe2 and Fe6, both of which have a free coordination site after the dissociation of the belt-sulfur atom S2B.
Understanding the biological system marks the starting point for an energy saving and sustainable alternative to the industrial process. The enzymatic system is a two-component metalloprotein (Av1 & Av2 for the two proteins isolated from the soil bacterium Azotobacter vinelandii) that contain two exotic metallocenters to achieve the elaborated electron transfer reaction that is needed to reduce N2. The active site of nitrogenase is the iron-molybdenum-cofactor (FeMo-cofactor, [7Fe:9S:C:Mo:R-homocitrate]), one of the most complex metalloclusters that can be found in Nature. How nitrogenase achieves catalysis is poorly understood, an instance that is intimately connected with the limited insights into structural and electronic states of the active site during turnover. A key question concerns how substrates interact with the FeMo-cofactor and are activated for the reduction process.
Carbon monoxide (CO) is a powerful inhibitor of the N2 reduction and was used to trap the system under turnover conditions. Due to the oxygen sensitivity of the metallocenters, crystals of the nitrogenase MoFe-protein (Av1) were grown under anaerobic conditions in the presence of CO. X-ray diffraction data to a resolution of 1.50 Å were collected at SSRL Beam Line 12-2. The resulting structure revealed for the first time a ligand-bound form of the nitrogenase active site. The binding geometry of CO is achieved by a displacement of one of the sulfur atoms (S2B) from the active site, leading to coordination of CO to two iron sites. Remarkably, S2B can be reversibly re-inserted as shown in the structure of the reactivated enzyme at a resolution of 1.43 Å. Given that S2B and CO have only 1 and 2 atoms, respectively, high resolution crystallography made possible by BL12-2 was essential to accurately define the binding interactions. The high X-ray intensity of BL12-2 at energies below the Fe-edge was also critical for these studies, since the appreciable anomalous scattering of S at these energies could directly establish that CO binding is accompanied by S2B displacement.
The structural data provide a first indication where and how substrates interact with FeMo-cofactor and suggest a possible route for the reduction of dinitrogen. The crystallographic data obtained at SSRL mark the starting point for the structural investigation of a variety of liganded forms of the enzymatic system that mimic different states in the N2 reduction process. This will allow detailed structural insights into the catalytic mechanism, as well as provide a structure-guided approach for spectroscopic and theoretical studies, that have the potential to directly “look at nitrogenase at work”.
T. Spatzal, K. A. Perez, O. Einsle, J. B. Howard and D. C. Rees, "Ligand Binding to the FeMo-cofactor: Structures of CO-bound and Reactivated Nitrogenase", Science 345, 1620 (2014), DOI: 10.1126/science.1256679.




