Concerns over the finite availability of oil and the effect of greenhouse gases on climate have spurred intense efforts to develop electric-drive vehicles; the major barrier to successful commercialization being battery technology. Although Li-ion batteries, crucial in the boom of portable electronics, stand as the technology of choice in soon-to-be marketed models, further improvements in their energy density, cost, cycle life and safety are still necessary1,2. Observation of the movement of chemical phase transition fronts and changes to the electrode pore structure, which allows effective wetting of the particles by the electrolyte and transport of lithium ions, could direct new strategies for design of next generation high energy density devices. Hence, monitoring changes in electrodes during battery operation (i.e., insertion/extraction of Li ions) requires imaging of morphological as well as chemical changes. XANES microscopy holds the promise of adding a new dimension, 3D nanoscale chemical and architectural visualization, to the diagnostics of Li-ion battery electrodes.
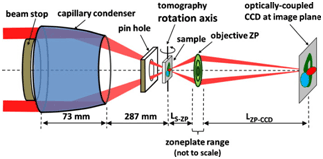
This work describes two recent publications in which X-ray absorption near edge structure (XANES) microscopy, a revolutionary technique based on the combination of full-field transmission X-ray microscopy (TXM; see Figure 1) with XANES, was used to obtain nanotomography on materials found in Li-ion battery electrodes (Nelson et al. 2011) and on the battery electrodes themselves (Meirer et al. 2011). The full-field transmission X-ray microscope on SSRL Beam Line 6-2 is capable of imaging from 4 to 14 keV, a range suitable for spectroscopic imaging of many metals used in battery electrodes and other materials.
With a field of view of 30 microns, extendable to millimeter rage with mosaic imaging, the microscope can be used to obtain single-pixel (15-30 nanometers) XANES spectra, resulting inapproximatelyone million XANES spectra per energy stack. Fitting of the XANES results in a chemical phase map at 30-nanometer resolution (see Figure 2 for schematic of the technique). Because this method combines high resolution with relatively large field of view and deep hard X-ray penetration of materials, it can provide 2D and 3D chemical information over relatively large areas relevant to hierarchical structures found in energy materials such as battery electrodes, fuel cells, and catalytic systems.
The potential impact of this technique is illustrated with the study of the changes taking place in NiO while cycled in a Li battery. NiO is considered an alternative anode material because of its very high charge storage capability3. The use of XANES microscopy to analyze Li-ion NiO battery electrodes at different states of charge results in a series of images in which the presence of NiO and Ni, the phase produced upon reduction, can be resolved and correlated with changes in morphology and porosity.
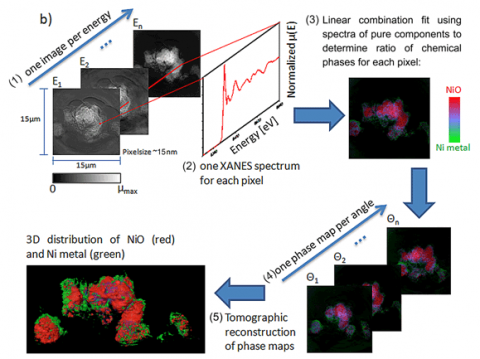
Within the framework of energy storage, this work adds a completely new dimension to the diagnostics of Li-ion battery electrodes, which are devices of great technological relevance because of their implementation in electric-drive vehicles. More generally speaking, 3D XANES microscopy is a unique technique that combines unprecedented spatial and energy resolution with large fields of view and fast acquisition (images can be obtained in minutes to a few hours) whose capabilities and high throughput lead to an overarching impact in a variety of fields as diverse as energy storage, archaeological objects, and biomaterials. Preliminary work on NiO/Ni imaging was published in Applied Physics Letters and the 3D XANES work on Li ion battery electrodes has been published in the Journal of Synchrotron Radiation. .
Acknowledgments:
Cabana acknowledges funding support by the Assistant Secretary for Energy Efficiency and Renewable Energy (Office of Vehicle Technologies of the U.S. Department of Energy) under Contract No. DE-AC02-05CH11231, and as part of the Northeastern Center for Chemical Energy Storage, an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy (Office of Science, Office of Basic Energy Sciences) under Award Number DE-SC0001294. Nelson et al.acknowledge financial support from a DOE-funded EFRC on Science Based Nano‐Structure Design and Synthesis of Heterogeneous Functional Materials for Energy Systems (HeteroFoaMCenter) under Contract No. DE‐SC0001061; from the National Science Foundation (Award CBET‐0828612); and the ASEE National Defense Science and Engineering Graduate Fellowship program.The transmission X-ray microscope at SSRL has been supported by the National Institutes of Health (NIH)/ National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant number 5R01EB004321. SSRL is a National User Facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
- Tarascon, J.M. & Armand, M., Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 414, 359-367 (2001).
- Goodenough, J.B. & Kim, Y., Challenges for Rechargeable Li Batteries Chem. Mater. 22 (3), 587-603 (2010).
- Poizot, P., Laruelle, S., Grugeon, S., Dupont, L., & Tarascon, J.M., Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries.Nature 407 (6803), 496-499 (2000).
F Meirer, J Cabana, Y Liu, A Mehta, JC Andrews, P Pianetta “3D imaging of chemical phase transformations at the nanoscale with full field transmission X-ray microscopy”J Synchrotron Rad 18 (2011).
GJ Nelson, WM Harris, JR Izzo, Jr., KN Grew, WKS Chiu, YS Chu, J Yi, JC Andrews, Y Liu, P Pianetta "Three Dimensional Mapping of Nickel Oxidation States using Full Field X-ray Absorption Near Edge Structure Nanotomography" Appl Phys Lett 98: 173109 (2011).




