Coherent X-ray Imaging Instrument
PURPOSE: This instrument will take advantage of the extremely bright, ultrashort LCLS pulses of hard x-rays to allow imaging of non-periodic nano-scale objects, including single biomolecules or small clusters, at or near atomic resolution.
X-ray scattering has long been used to determine atomic structures. The x-ray dose needed to achieve a given resolution for a particular sample can be calculated, and it is easy to show that the dose required to image a single molecule is much larger than the dose required to completely destroy the molecule through radiation damage processes. X-ray crystallographers mitigate this problem by spreading the damage over billions of molecules in a single crystal. Since the molecules are all identical and precisely aligned in the crystal, the x-ray scattering information is preserved and the structure can be determined.
LCLS offers another way around the damage problem. Since the FEL x-ray pulse is very intense and very short, it is possible in principle to deliver the required dose to a nano-scale sample and record the scattered x-ray information before the damage processes have time to destroy the sample. In other words, an LCLS x-ray pulse could be focused onto a single molecule, which would be destroyed – but not before the scattered x-rays are already on their way to the detector carrying the information needed to deduce the image. This technique offers the possibility of determining structures for samples which do not form crystals, including important classes of biological macromolecules.
The areas of study for this instrument will include:
- 3D imaging at or near atomic resolution of non-crystalline biomolecules (e.g. viruses, large protein molecular machines), nanoparticles (e.g. quantum dots), and amorphous and disordered materials..
- 3D structure determination of nanocrystals and ordered 2D structures (e.g. biomembranes)
- 3D characterization of strains and defects in crystals, dislocation and deformation structures.
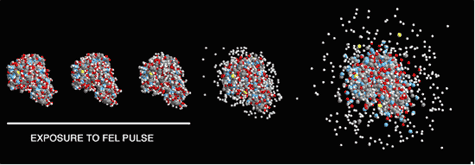
Calculation of the Coulomb explosion of a protein molecule (lysozyme) exposed to the focused pulse of an x-ray free-electron laser. With a very short x-ray pulse (indicated by the white line), atomic positions remain virtually unchanged during the exposure [from R. Neutze, R. Wouts, D. van der Spoel, E. Weckert, and J. Hajdu, Nature 406, 752 (2000)].
INSTRUMENT CONCEPT: A coherent pulse of x-rays illuminates the sample, producing a continuous diffraction pattern from a non-crystalline specimen. The diffraction pattern is recorded by a pixel array detector, which has high quantum efficiency and dynamic range. When such a coherent diffraction pattern is sampled at spacings sufficiently finer than the inverse of the sample size, the phase information can be directly retrieved by using an iterative process.
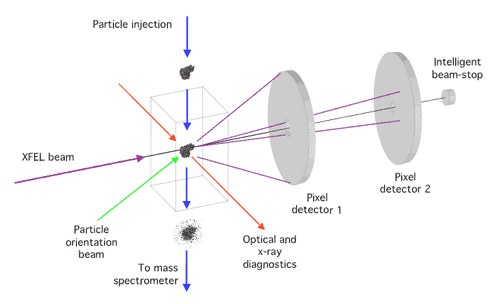
Schematic diagram of the single-particle diffraction imaging experiment at LCLS. Copies of a reproducible sample are exposed to the beam one by one in random orientations. 2D detectors record the coherent X-ray scattering. A 3D image of the sample can be reconstructed from the diffraction patterns.
The sample will be injected into high vacuum and into a tightly focused beam. A full 3D reconstruction will be performed by recording, pulse-by-pulse, and subsequently processing, a large number of diffraction patterns from the supply of reproducible samples.
UNIQUENESS: In combination with the LCLS, coherent x-ray imaging can potentially provide a new horizon of imaging nanoscale materials and large single macromolecules at or near atomic resolution in three dimensions. Resolution in these experiments would not depend on sample quality in the same way as in conventional crystallography, but would be a function of radiation intensity, pulse duration, wavelength, and the extent of ionization and sample movement during the exposures. The full peak brightness of the LCLS is fully exploited when imaging biological materials such as viruses and single macromolecules. The penetration depth of hard x-rays in combination with the coherent nature of the radiation will permit detailed 3D study of large, non-periodic structures, and provide capabilities that will go beyond conventional scanning probe microscopy, electron microscopy or x-ray crystallography.
