Many practical catalysts, illustrated by those used for fossil fuel conversion and vehicle exhaust clean-up, consist of expensive noble metals dispersed on high-area porous solid supports. When the metals are atomically dispersed and isolated from each other, they offer new properties and the highest efficiency of metal use—with each metal atom accessible to reacting molecules. But applications of supported noble metal catalysts are usually limited by the loss of accessibility of reactants to the metals that results from metal aggregation (sintering) during operation. Sintering can be suppressed by anchoring the metal atoms strongly to metal oxide supports, but strong metal–oxygen interactions often leave too few metal sites available for reactant binding and catalysis. When supported noble metal catalysts are exposed to reducing conditions at high temperatures, even those stably bonded to oxide supports ultimately sinter.
In recent work, catalyst performance data complemented by structure characterization data (including X-ray absorption spectra recorded at SSRL) show that (1) the benefits of anchoring can be enhanced by confining the atomically dispersed metal atoms on oxide nanoclusters that are dispersed as islands immobilized on a robust, high-surface-area support and that (2) these atomically dispersed metals are resistant to sintering, even at high temperatures. The strategy used for catalyst synthesis (Figure 1) was illustrated by grafting isolated and defective CeOx islands (called nanoglues) onto a SiO2 support and incorporating one platinum atom, on average, on each island host.
The catalyst activated by reduction in hydrogen became markedly more active for CO oxidation than the unactivated catalyst. The activated platinum remained atomically dispersed under both oxidizing and reducing environments at high temperatures, and was thus stable for long-term catalytic operation. The stability of the isolated catalytic platinum under reducing conditions is a result of the much stronger bonding of the platinum atoms to the CeOx islands than to the SiO2 support, which ensures that these atoms can move but remain confined to their respective islands. The strategy of using functional nanoglue islands to isolate and stably confine atomically dispersed metals and simultaneously enhance their reactivity is general, having been demonstrated for rhodium and palladium as well as platinum. The results suggest that the new class of stable atomically dispersed supported metal catalyst will take these materials a step closer to practical applications.
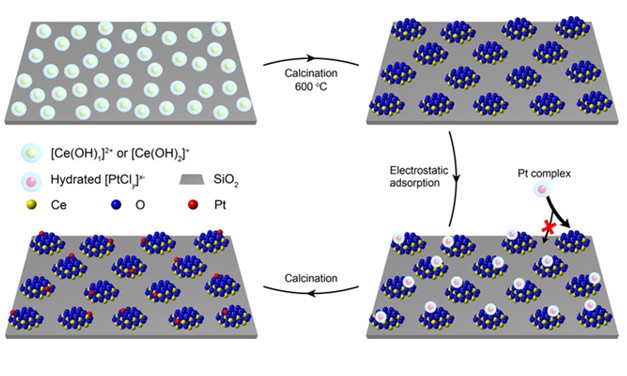
Figure 1. Schematic diagram illustrating the synthesis of functional CeOx nanoglue islands on a SiO2 support and incorporation of platinum single atoms selectively onto these islands. The islands bond strongly to the support and remain isolated from each other. In the selective synthesis of these islands, positively charged [Ce(OH)2]+ and [Ce(OH)]2+ are produced in a weakly alkaline aqueous synthesis solution by reaction of a precursor cerium salt with OH−—and these positively charged Ce-containing ions electrostatically adsorb onto the negatively charged SiO2 support. The resulting adsorbed precursors, during high-temperature calcination, self-assemble into CeOx nanocluster islands, observed by high-resolution electron microscopy. These islands act as nanoglues to localize catalytically active platinum atoms. Optimizing the aqueous solution pH in the second synthesis step, wherein the platinum atoms become anchored, causes the CeOx islands to be positively charged, so that negatively charged platinum-containing species selectively adsorb onto them and not the SiO2. Subsequent washing and calcination eliminate residues and leave the isolated platinum atoms bonded to the nanoclusters—where they are accessible to gas-phase reactants in high-temperature catalytic reactors and remain stably isolated to function for long times.
The work at SSRL was supported by DOE BES, and the work directed by Yong Wang at Washington State University and at PNNL, and the work directed by Bruce Gates at the University of California, Davis, was also supported by DOE BES.
X. Li, X. I. P. Hernandez, Y. Chen, J. Xu, J. Zhao, C. Pao, C.-Y. Fang, J. Zeng, Y. Wang, B. C. Gates, J. Liu, “Functional CeOx Nanoglues for Robust Atomically Dispersed Catalysts,” Nature (2022) DOI: 10.1038/s41586-022-05251-6

