Understanding the long-term evolution of uranium (U) mobility in contaminated soils and sediments is crucial for their management but little is known about potential transformations that could affect U-bearing phases over centennial or millennial time scales. Although reduced U forms (under oxidation state +IV) are known to be much less soluble than oxidized ones (under oxidation state +VI), it is now accepted that U(IV) often accumulates as non-crystalline species in sub-surface environments. Such non-crystalline phases are more soluble than crystalline U(IV) minerals such as uraninite (UO2), which strongly calls for a better knowledge on their possible transformations under natural conditions.
Within the framework of the France-Stanford program and of French EC2CO CNRS and IRSN projects, a team of researchers from IMPMC (CNRS-Sorbonne Université-MNHN-IRD), IRSN (the French Radiation Protection and Nuclear Safety Institute), EDYTEM (Université Grenoble Alpes-Université Savoie Mont Blanc-CNRS), IPGP (Université de Paris-CNRS) and SSRL, have studied the evolution of noncrystalline U(IV) along a sediment core from a naturally U-enriched Alpine lake from France (Lake Nègre, Mercantour-Argentera Massif).
Dating these lake sediments using 14C decay revealed that they deposited over 3,300 years in this small Alpine catchment. Moreover, these sediments display U enrichments comparable to U contaminated sites (350 to 760 µg/g) and can thus be considered as natural analogues for studying the long-term evolution of U in contaminated lake sediments. A combination of isotopic proxies ((234U/238U), δ238U and 238U/232Th) showed that U inputs and deposition modes in the lake remained similar over the 3,300-year period, indicating that, if any, potential U transformation would have occurred within the sediment after deposition (i.e. diagenetic process).
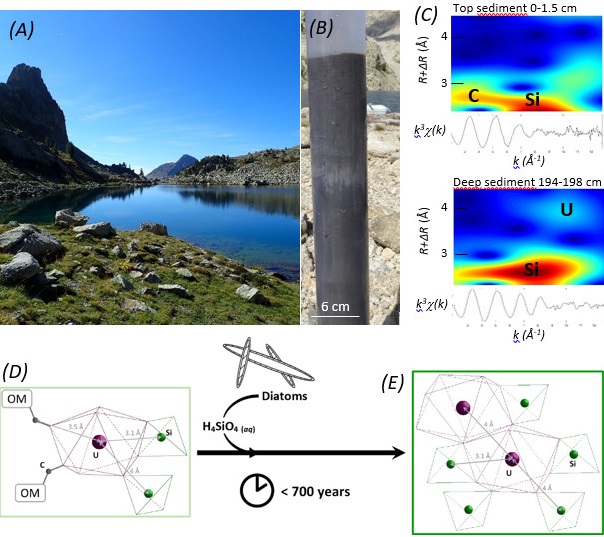
Synchrotron-based extended x-ray absorption fine structure (EXAFS) spectra at the U L3-edge were collected in fluorescence detection mode on the high-flux multipole wiggler beam line 11-2 at SSRL. This analysis allowed the team to resolve the molecular-level structure around U atoms in the sediment samples, along the lake sediment core.
The obtained results revealed that U was initially deposited as noncrystalline U(IV) species, mostly as isolated U(IV) ions bound to organic matter and silicate or phosphate moieties. After less than 700 years, U speciation clearly evolved to a new phase still present after 3,300 years. This new U molecular environment consists of U(IV) bound to silicate groups, with heavy atoms at long distance (~4 Å) interpreted as U neighbors. This speciation is interpreted as a continuum of U-Si polymers with a local structure close to that of coffinite (USiO4.nH2O) that formed thanks to the high dissolved silica availability in the pore waters. Further investigation of the deep sediments by scanning transmission electron microscopy (STEM-EDXS) allowed the detection of dispersed U and of only one U-rich nanoparticle, reinforcing this interpretation.
This study contributes to understand why the U(IV) mineral uraninite (UO2) is not as prevalent as originally supposed in natural anoxic environments. UO2 formation may be inhibited when high concentrations of U(IV)-binding ligands, especially silicate, are available with respect to dissolved U(IV). Here, the abundance of silica from diatom tests may have prevented uraninite precipitation and favored the persistence of noncrystalline U. Finally, this unexpected mineralogical transformation did not significantly improve U scavenging efficiency as inferred from chemical extractions, which has important implications for the management of U-contaminated sediments.
P. Lefebvre, A. Gourgiotis, A. Mangeret, P. Sabatier, P. Le Pape, O. Diez, P. Louvat, N. Menguy, P. Merrot, C. Baya, M. Zebracki, P. Blanchart, E. Malet, D. Jezequel, J.-L. Reyss, J. R. Bargar, J. Gaillardet, C. Cazala and G. Morin, "Diagenetic Formation of Uranium-silica Polymers in Lake Sediments over 3,300 Years", Proc. Natl. Acad. Sci. USA 118, e2021844118 (2021)doi: 10.1073/pnas.2021844118

