Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of unknown cause in which oligodendrocytes and myelin are targets of injury. The oligodendrocyte is the cell that produces the myelin that surrounds the axons of neurons, ensuring the rapid conduction of information from the central nervous system (CNS) to muscles, and from the sensory receptors to the CNS – giving rise to movements and sensations. Two cardinal features dominate the neuropathology of all MS lesions: loss of myelin (demyelination) and inflammation. Genetic, environmental and pathogenic factors contribute to immune cell activation in the peripheral circulation that trigger MS attacks as they invade the CNS (inflammation) and damage myelin and oligodendrocytes. Myelin becomes detached from axons (demyelination), and is eventually destroyed. Paralysis, blindness, numbness, bowel and bladder disturbances in MS result from this loss of nerve transmission.
Research has largely focused on identifying a single cause and therapy for all MS patients, but this approach fails to address the clinical, genetic and radiographic heterogeneity that underlies the variable response of MS patients to any given treatment. The definition of MS as a disease with CNS demyelinating lesions disseminated in space and time certainly sets the stage for gathering a wide variety of disorders under one all-inclusive name, and as early as 1982 it was postulated that MS may not be a single disease.1
In support of this hypothesis, a neuropathological study performed on a large collection of MS biopsies and autopsies has demonstrated that pathologic heterogeneity also exists in MS. This pathologic heterogeneity of MS lesions is apparent in the earliest stages of a patient’s lesion formation (early active lesions) and suggests that the targets of injury (myelin versus oligodendrocyte), and the mechanisms of demyelination, are different in different subgroups of the disease.2 Early active lesions can be classified into four types (called immunopatterns I, II, III and IV). Although pathologic heterogeneity is observed among MS patients, immunopatterns in multiple active lesions from a single patient are identical.3 Furthermore, patterns persist in tissue sampled at different time points from the same MS patient reinforcing the concept of patient-dependent immunopathological heterogeneity in early MS.3
These observations have potentially significant therapeutic implications, as individualized therapeutic approaches to treat the early stage of MS may need to be considered. In support of this hypothesis, pattern II MS, characterized by deposition of immunoglobulins and complement consistent with antibody-mediated demyelination, has been shown to be uniquely responsive to plasma exchange.4, 5 Because this pathological classification requires tissue collected at biopsy, the challenge has now become to identify surrogate magnetic resonance imaging (MRI), clinical, genetic, serological and/or cerebrospinal fluid markers that correlate with immunopatterns in the general non-biopsied MS population.
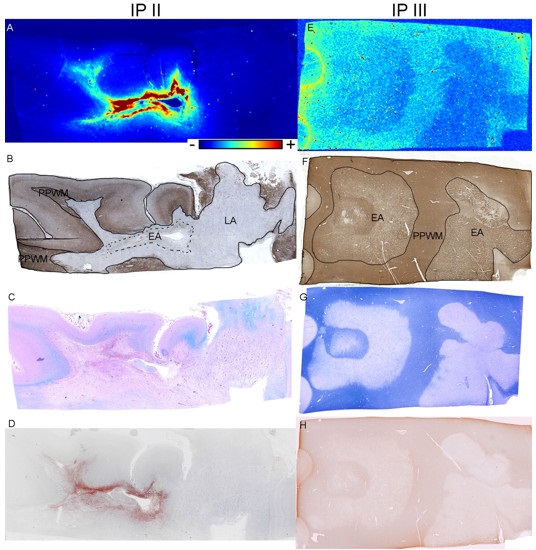
Figure. Iron in early active (EA) multiple sclerosis (MS) lesions. (A–C) Immunopattern (IP) II MS; (D–F) IP III MS. (A) Iron is increased in IP II EA lesions (X‐ray fluorescence imaging [XFI]). (B) The lack of proteolipid protein (PLP) immunoreactivity indicates demyelination; the various regions of interest are outlined and named (EA = early active lesion; LA = late active lesion; PPWM = periplaque white matter) on this map (PLP). (C) The lack of Luxol fast blue (Lfb) staining indicates demyelination (Lfb/hematoxylin and eosin [HE]). (D) Complement deposition is present in the EA lesion (C9neo). (E) Iron is decreased in IP III EA lesions (XFI). (F) PLP immunoreactivity is still present in the EA lesions; the various regions of interest are outlined and named on this map (PLP). (G) The lack of Lfb staining indicates demyelination (Lfb/HE). (H) There is preferential loss of myelin‐associated glycoprotein (MAG; compare with F; MAG). Color scales (A, E) represent the normalized total Kα fluorescence counts, proportional to total metal present, from blue (lowest) to red (highest). Scale bar = 3mm.
A collaborative research team (University of Saskatchewan, Mayo Clinic, Stanford Synchrotron Radiation Lightsource, University of Vienna, University of Göttingen) has used a multidisciplinary approach involving synchrotron x‐ray fluorescence imaging, histology, and immunohistochemistry to compare the quantity and distribution of iron between immunopatterns II and III (more than 80% of patients with acute MS). Using SSRL beam lines 10-2 and 2-3, we show that the distribution and content of iron are heterogeneous in different immunopatterns. Iron accumulates in macrophages in immunopattern II, but not immunopattern III lesions. This iron heterogeneity in the two most common MS immunopatterns may be explained by different macrophage polarization, origin, or different demyelination mechanisms.
Our novel observation that immunopattern II MS lesions contain significantly more iron than immunopattern III lesions, supports the concept of hetereogeneity in MS. It also suggests using the ratio of iron in the lesion to the iron in the surrounding tissue as a potential MRI stratification marker in MS and paves the way for developing new or using existing iron‐sensitive MRI techniques to differentiate among immunopatterns in the general non-biopsied MS patient population.
This study was funded by the Canada Research Chairs Program (Popescu BF), the Saskatchewan Health Research Foundation (Popescu BF), Biogen Idec (Lucchinetti CF, Popescu BF), and the National Institute of Neurological Disorders and Stroke (NINDS), NIH (Lucchinetti CF).
- S. Poser, N. E. Raun, and W. Poser, “Age at Onset, Initial Symptomatology and the Course of Multiple Sclerosis”, Acta Neurol. Scand. 66, 355 (1982)
- C. Lucchinetti, W. Bruck, J. Parisi et al., “Heterogeneity of Multiple Sclerosis Lesions: Implications for the Pathogenesis of Demyelination”, Ann. Neurol. 47, 707 (2000)
- I. Metz, S. D. Weigand, B. F. Popescu et al., “Pathologic Heterogeneity Persists in Early Active Multiple Sclerosis Lesions”, Ann. Neurol. 75, 728 (2014)
- M. Keegan, F. Konig, R. McClelland et al., “Relation between Humoral Pathological Changes in Multiple Sclerosis and Response to Therapeutic Plasma Exchange”, Lancet 366, 579 (2005)
- L. Stork, D. Ellenberger, T. Beissbarth et al., “Differences in the Reponses to Apheresis Therapy of Patients with 3 Histopathologically Classified Immunopathological Patterns of Multiple Sclerosis”, JAMANeurol. 75, 428 (2018)
M. Tham, J. M. Frischer, S. D. Weigand, P. D. Fitz-Gibbon, S. M. Webb, Y. Guo, R. C. Adiele, C. A. Robinson, W. Bruck, H. Lassmann, K. L. Furber, M. J. Pushie, J. E. Parisi, C. F. Lucchinetti and B. F. Popescu, "Iron Heterogeneity in Early Active Multiple Sclerosis Lesions", Ann. Neurol. 89, 498 (2021) doi: 10.1002/ana.25974




