The metal copper (Cu) is known to be an essential trace element for many organisms but it is also considered a severe contaminant at higher concentrations. Especially in soils with changing redox conditions, Cu binding mechanisms and, thus, Cu mobility are hard to predict. The metal is known to have a high affinity to soil organic matter (SOM), i.e., it can either be sequestered by adsorption to solid-phase organic matter or mobilized by complexation with dissolved organic matter. Under reducing conditions, Cu(II) can also be reduced to Cu(I) via biotic and abiotic processes and precipitate in the form of sulfidic minerals.
Researchers from Bayreuth University, in collaboration with scientists from ETH Zürich, SLU Uppsala, and Stanford University, recently studied the behavior of Cu along a natural gradient in redox conditions. They investigated the soil around a so-called mofette, which is a natural, diffuse exhalation site of cold carbon dioxide. Such sites are usually associated to (post-) volcanic processes and they occur in many seismically active regions all over the world. Mofette soils are very suitable natural laboratories since they allow studying a redox gradient from permanently anoxic soil conditions in the degassing center (with almost 100% carbon dioxide in soil air) to oxic conditions within only some meters from the mofette. Due to the decreased oxygen availability, litter decomposition is inhibited and poorly decomposed SOM accumulates. Thus, in addition to the gradient in redox conditions, mofettes can exhibit gradients in both SOM content and SOM composition.

Figure 1. Picture of the investigated mofette site (left) and Cu sorption isotherms determined for mofette, transitions, and reference soil in a Cu spike experiment (right). Reprinted with permission from Mehlhorn et al. 2018, ES&T, DOI: 10.1021/acs.est.8b02668, Copyright 2018 American Chemical Society.
For their study, the researchers determined total Cu contents in soil and pore water samples from a transect over a wet mofette site in the Czech Republic. In addition, they conducted Cu adsorption experiments with natural soil samples from this transect, applying spikes of up to 11.7 mmol Cu kg‑1. Interestingly, the lowest Cu soil contents and highest Cu pore water concentrations, i.e., the highest Cu mobility, were determined in the transition soils with <10% oxygen, located between the degassing center (mofette) and the oxic reference soil. These were also the soils, which showed the lowest adsorption in the Cu spike experiments (Fig.1). The non-carbon-dioxide-influenced reference soils showed a higher Cu adsorption compared to the transition soils, and Cu soil contents were also increased directly in the mofette center. However, in samples from the degassing center, pore water Cu concentrations were close to detection limit, and Cu adsorption in the spike experiments significantly exceeded those of all other soils.
To identify the processes that caused the high Cu sequestration in mofettes compared to in the transition and reference soils, the authors investigated Cu solid-phase speciation in natural and Cu-spiked samples using x-ray absorption spectroscopy (XAS) at SSRL Beam Lines 4-1 and 4-3. They could show that in the transition and reference soils, Cu was predominantly present as Cu(II) bound via oxygen or nitrogen-containing groups to SOM. Also, the Cu that was added during the adsorption experiment was predominantly adsorbed to SOM. In the degassing center, however, almost three-fourths of the Cu were present in form of Cu sulfides. Also a large fraction of the spiked Cu(II) was reduced and precipitated as sulfidic minerals in addition to the adsorption to SOM.
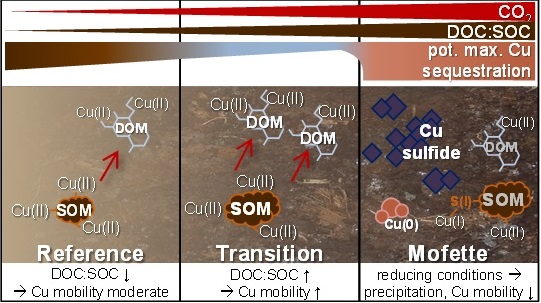
Figure 2. Conceptual model of Cu binding and mobility along the natural gradient of redox conditions and SOM at a mofette site. Reprinted with permission from Mehlhorn et al. 2018, ES&T, DOI: 10.1021/acs.est.8b02668, Copyright 2018 American Chemical Society.
Reduction of Cu(II) to Cu(I) under permanently anoxic conditions and following precipitation with sulfide can thus explain the effective Cu sequestration in mofettes. The different Cu mobility in transition and reference soils, however, could not be attributed to different sequestration mechanisms according to XAS. The accumulation of less decomposed SOM under anoxic soil conditions led to higher concentrations of dissolved organic matter in the carbon dioxide-influenced soils. This indicates that more Cu was complexed by dissolved organic matter, resulting in an increasing Cu mobility with increasing carbon dioxide. However, under permanently anoxic conditions, which occur in the degassing center, precipitation of sulfidic minerals effectively sequestered Cu from solution, leading to low Cu mobility despite high dissolved organic matter concentrations (Fig. 2).
The results obtained at the natural redox gradient of this mofette site are useful for risk assessment at Cu-contaminated sites. They indicate that the risk of an increased Cu mobility is highest in transition zones between anoxic and oxic soil conditions.
J. Mehlhorn, J. Besold, J. S. Lezama Pacheco, J. P. Gustafsson, R. Kretzschmar and B. Planer-Friedrich, "Copper Mobilization and Immobilization along an Organic Matter and Redox Gradient—Insights from a Mofette Site", Environ. Sci. Technol. 52, 13698 (2018) doi: 10.1021/acs.est.8b02668

