Energy storage devices have revolutionized the modern electronics industry by enabling the widespread application of portable electronic devices. Moreover, these storage devices also have the potential to reduce the dependence on fossil fuels by implementing electric vehicles in the market. To date, lithium ion batteries have dominated the market because of the high energy density delivered by them. However, one should look into the sustenance of such devices because Li is not one of the most abundant metals on Earth’s crust. Thus, developing an alternative to lithium ion batteries has become one of the key issues to ensure the sustainable future of energy storage devices. Sodium ion batteries provide one such alternative. Out of all the components of a battery, cathode materials play one of the key roles in determining the overall performance of such batteries. Unfortunately, sodium-ion batteries have been lagging behind their lithium ion counterpart in terms of performance. Thus, new design strategies must be undertaken in order to improve the performance of cathode materials for sodium ion batteries.

Figure 1. Three-dimensional elemental associations of pristine Na0.9Cu0.2Fe0.28Mn0.52O2 studied through transmission x-ray tomography. a) Visualizing the surface elemental associations at different angles with different colors corresponding to different association, and b) 2D cross-sectional association maps showing the bulk elemental associations. [Energy Environ. Sci., DOI: 10.1039/C8EE00309B (2018)]
Conventionally, homogeneous distribution of the transition metals has been the preferred route of synthesizing cathode materials for alkali metal ion batteries. Counterintuitively, a research team led by Dr. Feng Lin of Virginia Tech and Dr. Yijin Liu of SSRL has demonstrated that the heterogeneous distribution of the transition metals can also yield excellent electrochemical performance. In a research article published this year in Energy & Environmental Science, they have reported a compositionally heterogeneous Na0.9Cu0.2Fe0.28Mn0.52O2 material (Figure 1) with superior electrochemical performance. The researchers explored the compositional distribution of the transition metals through transmission x-ray microscopy (TXM) imaging. Moreover, the redox behavior of the transition metals as a function of depth was studied with the aid of soft x-ray absorption spectroscopy (soft XAS) and hard x-ray absorption spectroscopy (hard XAS). Batteries with such material yielded very high specific capacity (125 mAh/g at C/10 rate) with very stable capacity retention (no capacity fading after 100 cycles). Such superior stability of the battery performance has rarely been reported for sodium ion batteries. This design strategy has opened up plenty of room in the bottom for tuning the nano/mesoscale distribution of the transition metals in order to achieve superior battery performance.
In addition to demonstrating the effectiveness of compositional heterogeneity for good battery performance, the researchers have also explored the capacity fading mechanism of this material. TXM imaging on cycled materials (at 1C rate after 400 cycles) has shown that Mn segregation and migration onto the surface of the cathode particles may account for the capacity fading on long-term cycling. Thus, this study is not only significant in terms of providing a new cathode design strategy, but also enlightening the path towards further stabilization of layered oxide cathode materials.
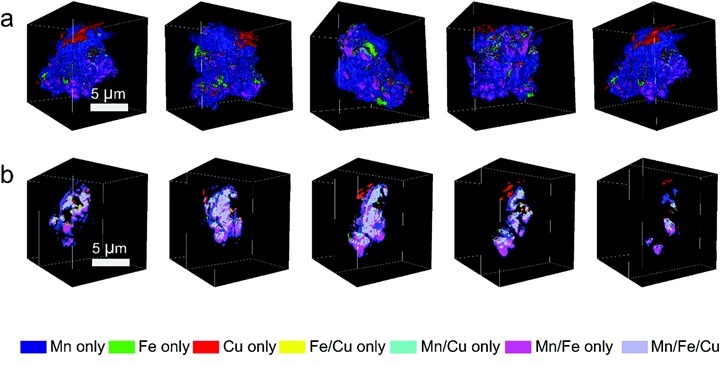
Figure 2. Visualizing elemental associations on the surface and in the bulk of Na0.9Cu0.2Fe0.28Mn0.52O2 after long term cycling. a) Surface elemental association maps of the cycled particles from different angles. Comparing with Figure 1, it can be seen that the surface is much more populated with the Mn-only association after long term cycling, and b) 2D cross-sectional elemental association maps from different depth in the particle visualizing the bulk elemental associations. [Energy Environ. Sci., DOI: 10.1039/C8EE00309B (2018)]
M. M. Rahman, Y. Xu, H. Cheng, Q. Shi, R. Kou, L. Mu, Q. Liu, S. Xia, X. Xiao, C.-J. Sun, D. Sokaras, D. Nordlund, J.-C. Zheng, Y. Liu and F. Lin, "Empowering Multicomponent Cathode Materials for Sodium Ion Batteries by Exploring Three-dimensional Compositional Heterogeneities", Energy Environ. Sci. (2018) doi: 10.1039/C8EE00309B

