Copper oxide is a widely used adsorptive material that removes trace amounts of H2S from various process streams via chemical reaction to form copper sulfide. At room temperature the thermodynamics favor a near complete conversion of CuO to copper sulfide in the presence of H2S. However, in application, the extent of conversion of the CuO to copper sulfide during reaction can be influenced by many factors, including the initial crystalline state of the CuO, and the rate at which solid products accumulate on the reactive surfaces or within pores of the CuO particles. This incomplete utilization of CuO is problematic for industrial applications because it typically leads to oversized equipment and/or frequent process shutdowns. Developing fundamental insight at the atomic scale for this reaction could overcome these limitations by providing a rational basis for the design of new materials and by leading to predictive models that allow for current materials to be operated toward their thermodynamic limits. Thus, experiments that combine reaction kinetic testing while also simultaneously capturing chemical and structural changes in the solid phase at multiple length scales are necessary to elucidate the fundamentals of these reactions at various length scales.
Previous studies were successful in semi-quantitatively relating properties of materials to performance in fixed-bed systems, however, differences in performance were often attributed to physical properties at the >10 mm scale (e.g., surface area, pore volume, bulk density). The effects of molecular scale material characteristics (e.g., microscopic shape, metal oxide crystallite size, and surface composition) were rarely investigated, thus, it is difficult to extend the conclusions from these studies across a broad range of conditions and materials
A research team led by Prof. Dante Simonetti (UCLA), Dr. Yijin Liu, and Dr. Simon Bare (SSRL) studied the reaction of CuO materials with similar pore structures and chemical composition using transmission x-ray microscopy (TXM) at Beam Line 6-2c and Cu K-edge x-ray absorption spectroscopy (XAS) at Beam Line 2-2 of SSRL (Figure 1). In-situ XAS experiments showed that materials with smaller CuO crystallites exhibit slower rates of reaction, which delays the covering of reactive surfaces and leads to higher overall conversion to copper sulfide products (Figure 1B). However, these XAS studies also showed that the local structure of the CuO samples were similar, indicating that other factors (i.e., the structure of the CuxSy product) also influence reaction rates and conversions. In-situ TXM experiments on individual particles revealed that the average kinetics within single particles are similar to the reaction kinetics measured from fixed bed of sorbents (Figure 1B). However, these experiments also showed that reaction fronts proceed heterogeneously within solid particles indicating significant diffusion impacts at length scales less than 10 µm (Figure 1A).
This combined characterization-kinetic analysis quantitatively demonstrated, for the first time, that CuO samples with similar chemical composition and pore structure can exhibit different H2S removal performance and that explaining these differences must include more detailed molecular descriptions. Furthermore, these studies highlight the need for more detailed study of (i) the exact structures of the CuxSy products, (ii) the nuisances that changing crystallite size and adding dopant atoms impart on CuO structure, and (iii) the quantitative evolution in 3 dimensions of the void spaces within the particle during reaction. Future in-situ sulfur XAS studies at Beam Line 4-3 and 3-D TXM and x-ray microtomography studies at 6-2c will seek to provide insight into these topics, thereby advancing the fundamental understanding of gas-solid CuO-H2S reactions.
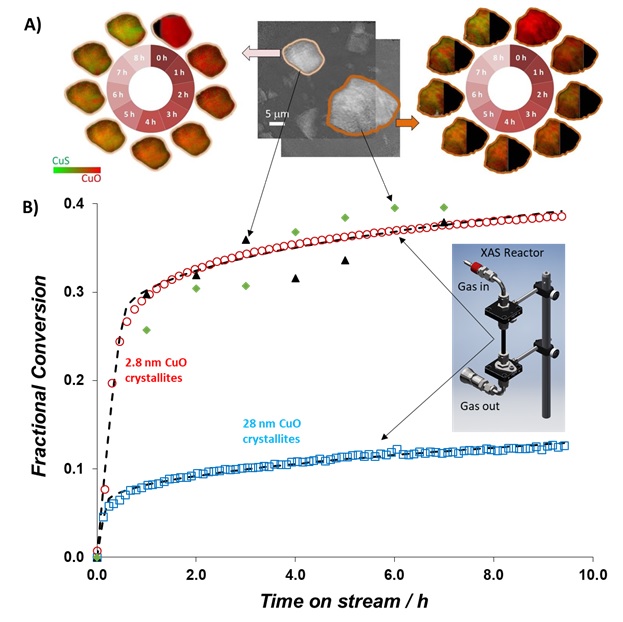
Figure 1. (A) CuO and CuS concentration maps derived from XANES analysis of TXM images of individual CuO particle during reaction with 1000 ppm H2S. (B) Fractional conversion versus time (derived from linear combination fitting of Cu K-edge XANES) of fixed beds of CuO particles consisting of 2 different crystallite sizes (red circles are 2.8 nm and blue squares are 28 nm) and of individual CuO particles.
A. S. Hoffman, S. Azzam, K. Zhang, Y. Xu, Y. Liu, S. R. Bare and D. A. Simonetti, "Direct Observation of the Kinetics of Gas–Solid Reactions Using in Situ Kinetic and Spectroscopic Techniques", React. Chem. Eng. (2018) doi: 10.1039/C8RE00020D

