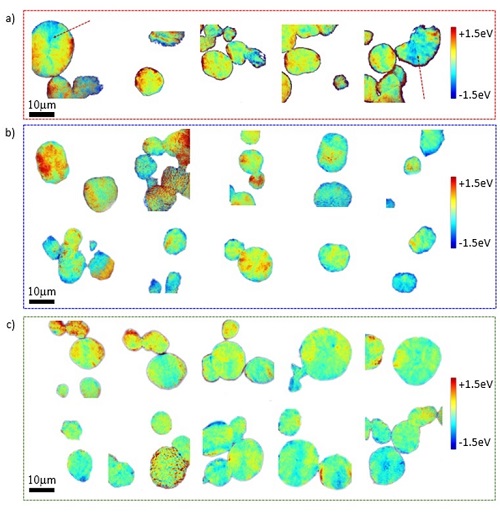
State-of-charge (SOC) inhomogeneities, which are often observed in electrodes undergoing charge or discharge in Li-ion batteries, could be caused by many factors, including the cell geometry, electrode thickness, porosity and cycling rate/history. Both electronic wiring and ionic diffusion pathways play key roles in affecting the non-equilibrium lithium distributions. In addition to inhomogeneity at the electrode level, SOC non-equilibrium has also been observed in individual secondary particles and large primary single crystal particles, highlighting the complexity in the battery’s local chemistry over a wide range of length scales. These heterogeneities are of important consequences for the operation of electrochemical cells because they can lead to loss of capacity, local over-charge or over-discharge, or unsafe conditions. Thus, understanding the formation mechanism of the non-uniformity is fundamentally interesting and practically important.
To study heterogeneity at the particle level, active materials are often prepared through either electrochemical process or chemical process, which have their own pros and cons. The electrochemical process is the reaction in real batteries and is, thus, most relevant to real-world applications. However, the structural complexity in real battery electrode adds practical difficulties to many experiments. On the other hand, in the chemical delithiation process, the battery particles are homogeneously exposed to the oxidant, avoiding any artifacts caused by non-uniform electronic wiring and electrolyte wetting. It is, however, still debatable how well the chemical delithiation process can represent the true electrochemical cycling process occurring in a real battery cell. Nevertheless, studying the chemically delithiated compounds can provide valuable insights into the role that other cell components play in degradation processes.

Figure 2. Ni L-edge XAS spectra of (a) pristine LiNi0.6Mn0.2Co0.2O2; (b) chemically delithiated NMC-622; (c) electrochemically charged NMC622 electrode and (d) electrochemically discharged NMC-622 electrode collected using TEY (solid black curve) and FY (dashed red curve) modes.
A research team led by Drs. Marca Doeff (LBL), Yijin Liu and Dennis Nordlund (SSRL) compared pristine, chemically delithiated, and partially charged or discharged particles to evaluate the SOC heterogeneity using cutting edge synchrotron techniques at SSRL. They used the transmission x-ray microscopy at Beam Line 6-2c of SSRL to evaluate the chemical heterogeneity in the bulk of the particles. The surface chemistry of these samples was probed using soft x-ray spectroscopy at Beam Line 8-2 of SSRL. Their results show that while the chemically delithiated samples can represent the electrochemically charged samples in terms of mesoscale SOC heterogeneity in large polycrystalline particle ensembles, the surface chemistry (e.g., surface reconstruction) shows major differences due to the presence of cathode-electrolyte reactions in practical cells.
This in-depth study highlights the fact that the local chemistry in battery systems could be very different from that anticipation at the bulk level. Understanding of the interplay between different materials components at different length scales could critically inform the design of next generation battery material and chemistry.
C. Tian, Y. Xu, D. Nordlund, F. Lin, J. Liu, Z. Sun, Y. Liu and M. Doeff, "Charge Heterogeneity and Surface Chemistry in Polycrystalline Cathode Materials", Joule 2, 464(2018) doi: 10.1016/j.joule.2017.12.008

