Traditional heterogeneous catalysts contain a range of particles sizes, crystallographic faces, and surface structures. This heterogeneity makes catalysts design a challenge because the active sites responsible for catalytic activity are simply not known. An emerging class of “atomically–precise” nanocatalysts can help alleviate this problem because they form with precisely-known crystal structures. This leads to a unique situation where experimental and computational researchers can study nearly identical systems and unambiguously identify active surface sites. The hope is that once highly catalytic surface sites have been identified then synthetic strategies can be developed to produce new catalyst materials with a high density of desirable surface sites.
Electrochemical CO2 conversion and H2 evolution are promising candidates for renewable fuels synthesis. These two reactions both require that water is oxidized into O2 at the anode. The so-called oxygen evolution reaction (OER) is problematic for both large-scale deployment of both CO2 conversion and H2 evolution systems because it requires large amounts of energy. Another problematic aspect of OER is that typical catalysts contain extremely expensive precious metals like Pt, Ir or Ru. Current research interests have focused on identifying inexpensive, earth abundant OER catalysts with performance that exceeds traditional precious-metal based catalysts. However, heterogeneity in typical OER catalyst samples makes identification of the true OER active site challenging, and the literature contains a range of reported reaction rates, reaction mechanisms, and active site structures.
In a recent ACS Catalysis publication researchers from the National Energy Technology Laboratory (NETL) described a new atomically-precise OER catalyst with performance exceeding Pt, Ir and bulk NiO. Single-crystal x-ray crystallography revealed the catalyst contains a hexagonal ring of six nickel atoms that are capped with twelve organo-thiol ligands (Figure 1a). Electrochemical techniques showed the atomically-precise Ni6(PET)12 catalyst produced higher OER current at moderate overpotentials when compared with Pt, Ir and bulk NiO (Figure 2a). Long term testing showed good stability, and Ni6(PET)12 and Ir required equivalent potentials to sustain an OER turnover frequency of 10 molecules per second per active site. X-ray absorption spectroscopy was used to study the electronic structure of the atomically-precise Ni6(PET)12 catalyst compared with bulk Ni and NiO, and subtle differences were identified between Ni6(PET)12 and bulk NiO that stem from the Ni atoms being coordinated with either S or O atoms. Density functional theory (DFT) was used to model the entire OER process at Ni6(PET)12 to identify likely reaction centers, potential determining steps, and kinetically limiting steps within the reaction mechanism. This work shows that atomically-precise catalysts allow unambiguous determination of catalytic active sites and reaction pathways, and their continued use will help develop the next generation of catalysts for OER and other important reactions.
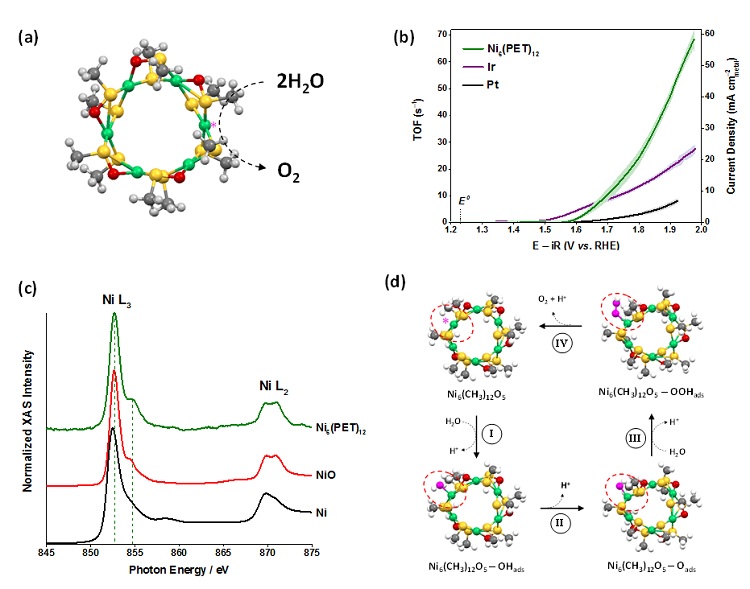
Figure 1. (a) Ni6(PET)12 crystal structure showing OER at the proposed active site. (b) Electrochemical oxygen evolution reaction performance of Ni6(PET)12 compared with traditional Pt and Ir catalysts. (c) X-ray absorption spectroscopy of Ni6(PET)12 compared with bulk NiO and Ni metal. (d) Density functional theory (DFT) predicted reaction mechanism on the atomically-precise catalyst. Adapted from the primary citation listed below.
D. R. Kauffman, D. Alfonso, D. N. Tafen, J. Lekse, C. Wang, X. Deng, J. Lee, H. Jang, J.-s. Lee, S. Kumar and C. Matranga, "Electrocatalytic Oxygen Evolution with an Atomically Precise Nickel Catalyst", ACS Catal. 6, 1225 (2016), DOI: 10.1021/acscatal.5b02633.




