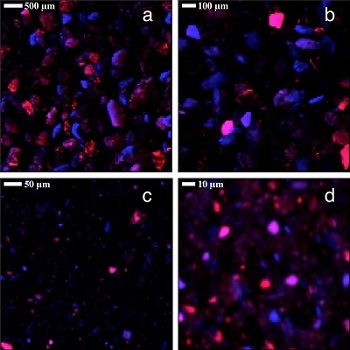
Figure 1. Micro-XRF maps of the (a) 250-500 µm, (b) 75-125 µm, (c) 32-45 µm, and (d) <20 µm size fractions of the Descarga mine tailings sample. Arsenic and iron Ka fluorescence signals are shown in red and blue, respectively; purple hues indicate particles that contain both elements.
Residential developments near former mining regions in southern California face potential environmental and health risks due to the weathering of arsenic-bearing deposits and mine wastes. Fine-grained particles possessing elevated concentrations of arsenic from these areas can be easily transported by water and dispersed by wind. Understanding the speciation of arsenic is essential to evaluating mobility and geoavailability because certain species of arsenic are more toxic and mobile than others. Speciation involves identifying the composition and relative proportions of chemical compounds present within a sample.
In a recent study, researchers of Chapman University, the U.S. Geological Survey and SSRL have investigated the speciation of arsenic changes within mine tailings and surrounding soil samples. The scientists identified arsenic-containing mineral and sorbed species in size-fractionated mine wastes and adjacent soils from the Solomon Mine and the Descarga Tailings Dam in the Randsburg Historic Mining District, California, using extended x-ray absorption fine structure (EXAFS) spectroscopy at SSRL’s Beam Line 11-2. These analyses were paired with micro-x-ray fluorescence (μXRF) mapping of separate size fractions to identify multiple populations of particles with different arsenic-to-iron ratios. Micro-XRF experiments were performed at SSRL’s Beam Line 2-3 and the Advanced Photon Source of the Argonne National Laboratory.
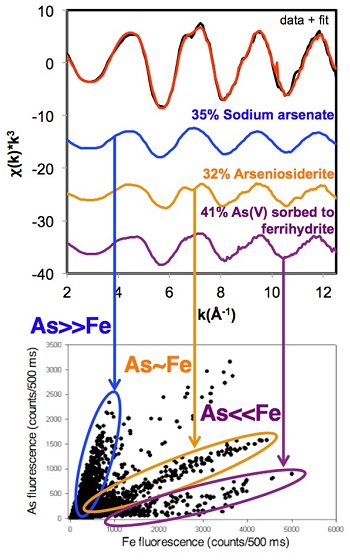
Figure 2. Linear combination fitting results (top) and associated µXRF arsenic-iron correlation scatterplot (below) for the ≤20 µm size fraction of a Descarga mine tailings sample show the associations between the µXRF and EXAFS speciation analyses.
Arsenic showed an inverse relationship between particle size and concentration, which would result from arsenic persisting in insoluble phases during weathering or forming sorbed species to fine-grained materials. Micro-XRF analyses confirmed that arsenic and iron are often found together (Figure 1), supporting macroscopic geochemical data showing a positive correlation between arsenic and iron. EXAFS spectroscopy identified several arsenic-containing phases including arseniosiderite, Ca2Fe33+(AsO4)3O3•3H2O, sodium arsenate (Na2HAsO4) – likely a proxy for similarly structured iron-free arsenate phases – and As5+ sorbed to iron oxyhydroxides; these species correlated well with the different As:Fe ratios apparent in µXRF studies of the same sample (Figure 2).
An inverse relationship between sodium arsenate and sorbed As5+ exists among the mine tailings samples, while the adjacent soil samples contain primarily sorbed arsenic. These differences in arsenic speciation between the mine tailings and the soil samples suggest that weathering of crystalline arsenic-bearing phases in tailings leads to sorption of dissolved arsenic to iron-oxyhydroxides in contaminated soils.
The detailed determination of chemical speciation illustrates the importance of applying a multi-technique approach for identifying metal(loid) species by using both physical and chemical methods. The results also encourage further quantitative studies of arsenic and other potentially hazardous elements at former mining sites.
C. S. Kim, C. Chi, S. R. Miller, R. A. Rosales, E. S. Sugihara, J. Akau, J. Rytuba and S. M. Webb, "(Micro)spectroscopic Analyses of Particle Size Dependence on Arsenic Distribution and Speciation in Mine Wastes", Environ. Sci. Technol. 47, 8164 (2013) doi: 10.1021/es4010653

