The bonding arrangement in amorphous materials plays a dominant role in determining their electrochemical, optical and transport properties. However, it remains a challenge to manipulate amorphous structures in a controlled manner. Recently, scientists at the Molecular Foundry at Lawrence Berkeley National Laboratory (LBNL) developed synthetic protocols for incorporating well-defined nanocrystals into amorphous materials [1,2]. This “nanocrystal-in-glass” approach not only allows combining two functional components in one material, but it could also provide a handle, by virtue of the interfacial covalent bond, for manipulating the glass structure and deliberately changing its properties.
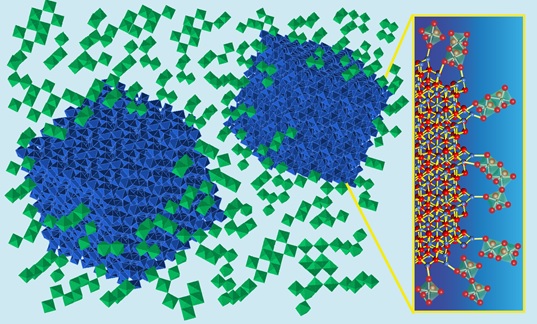
Now, researchers from LBNL and the Institute of Materials Science of Barcelona (ICMAB, Spain) have demonstrated the power of this approach by introducing tin-doped indium oxide nanocrystals (ITO NCs) into niobium oxide glass (NbOx) and showing that a new amorphous structure is created as a consequence of linking the glass to the nanocrystals (Figure 1).
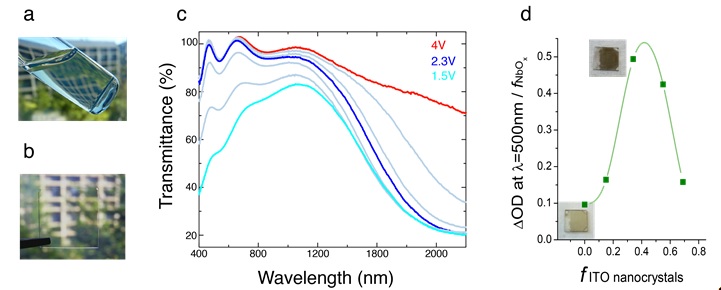
The two components of the composite, ITO NCs and NbOx, were selected for their different spectral-selective electrochromic properties. When electrochemically reduced, ITO NCs block near-infrared (NIR) light through a plasmonic electrochromic effect [3], while NbOx mostly blocks visible light. By linking these functional components, a previously unrealized optical switching behavior is achieved. Namely, these transparent ITO-in-NbOx films (Figure 2b) are capable of selectively blocking NIR and visible light by varying the applied electrochemical voltage over a range of 2.5 V (Figure 2c). In addition, the reconstructed NbOx glass has superior properties – its optical contrast is enhanced five-fold (Figure 2d) and it has excellent electrochemical cyclability, with 96% of charge capacity retained after 2,000 cycles. Window coatings with such a unique optical switch could reduce energy consumption in buildings by dynamically and independently modulating daylight and solar heat.
A key aspect of the research study was to control the composite structure at different length scales that govern the macroscopic electrochromic properties – from the atomic scale (bonding and crystal structure) to the mesoscale (crystallite sizes, interface densities and connectivity). The LBNL researchers controlled these characteristics by using a building-block colloidal approach, where nanocrystals and glass-forming molecules are synthesized separately and then linked together. In their colloidal approach, interfacial chemical bonds were made by combining ligand-stripped ITO NCs and polyniobate clusters (POMs) in aqueous solution. This process resulted in POM-stabilized nanocrystal dispersions, from which films were deposited and then thermally annealed.
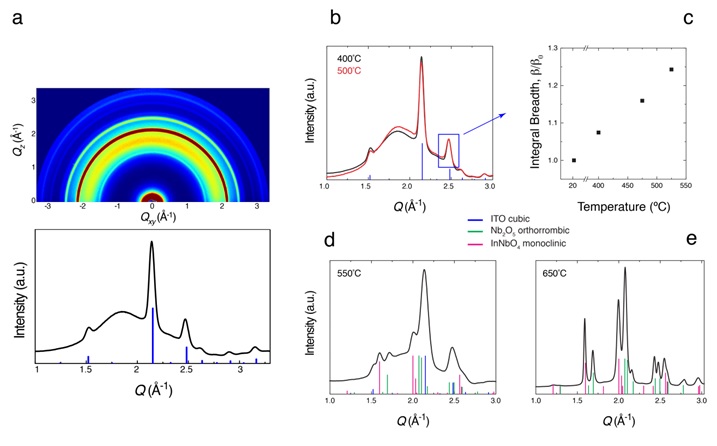
Grazing incidence x-ray diffraction (XRD) measurements, performed at SSRL’s Beam Line 11-3, enabled studies of the composite film structure (Figure 3). XRD patterns of films annealed at 400-500ºC show that the molecular POMs thermally condense, leading to an interconnected amorphous NbOx matrix that remains bonded to the crystalline ITO nanocrystals (Figure 3 a,b). Profile-fitting analysis enabled quantification of the broadening of the ITO Bragg peaks following thermal processing at different temperatures. The integral breadth monotonically increased with increasing temperature (Figure 3c), pointing to progressive interfacial dissolution of ITO in the adjacent NbOx matrix. At higher temperatures ion mobility increases and eventually a crystalline ternary InNbO4 phase is formed at 650ºC (Figure 3 d, e). This observation further supports that, at lower temperatures, indium and likely tin can locally diffuse into the amorphous NbOx matrix and disrupt its bonding arrangement.
To identify the structural changes in the NbOx amorphous network, the authors performed Raman spectroscopy on pure amorphous NbOx and ITO-in-NbOx with different nanocrystal-glass interfacial densities. The results showed that the degree of connectivity of the reconstructed NbOx network is decreased and the topology is dominated by highly distorted edge-sharing [NbO6] octahedral units as compared to the more regular, vertex-sharing topology observed in pure NbOx. Hence, the structure of the NbOx glass is profoundly altered by covalent linking to ITO nanocrystals and it becomes highly distorted, less interconnected and interfacially doped (Figure 1).
This reconstructed amorphous structure plays a crucial role in enhancing the electrochromic properties of NbOx. The authors suggest that the enhancements in both optical contrast and cyclability are related to the more open network structure, which facilitates ion insertion and extraction and helps relax the stress induced by these electrochemical processes. Consistent with this hypothesis, the charge capacity increased proportionally with the optical contrast.
Beyond electrochromics, this work shows that combining colloidal nanocrystals and glasses in a single heterogeneous composite is a powerful approach to create complex functionalities. Ideally, one could mix and match desired properties by selecting specific compositions. Through the interfacial bonding between the components, one could modify their structure and tune their optical, electrochemical and transport properties.
[1] R. Tangirala, J. L. Baker, A. P. Alivisatos and D. J. Milliron, “Modular Inorganic Nanocomposites by Conversion of Nanocrystal Superlattices”, Angew. Chem.-Int. Ed. 49, 2878 (2010).
[2] A. Llordes, A. T. Hammack, R. Buonsanti, R. Tangirala and S. Aloni et al., “Polyoxometalates and Colloidal Nanocrystals as Building Blocks for Metal Oxide Nanocomposite Films”, J. Mater. Chem. 21, 11631 (2011)
[3] G. Garcia, R. Buonsanti, E. L. Runnerstrom, R. J. Mendelsberg and A. Llordes et al., “Dynamically Modulating the Surface Plasmon Resonance of Doped Semiconductor Nanocrystals”, Nano Lett. 11, 4415 (2011).
A. Llordes, G. Garcia, J. Gazquez and D. J. Milliron, “Tunable Near-infrared and Visible Light Transmittance in Nanocrystal-in-Glass Composites”, Nature 500, 323 (2013) DOI: 10.1038/nature12398.

