The primary anthropogenic source of mercury (Hg) emissions into the atmosphere is coal-fired power utilities. This work explores materials designed for Hg capture to be applied in the ductwork of a power plant to prevent Hg release into the atmosphere. Bench-scale combustion experiments have been carried out, in which sorbent materials were placed in a simulated flue gas stream doped with ppb levels of Hg. The sorbent surfaces were probed using x-ray absorption spectroscopy to determine the mechanism of Hg binding and to ultimately improve solvent design. The spectroscopy data was analyzed alongside results from density functional theory (DFT) for benchmarking so that DFT can be used as a screening tool for material improvement and new design.
Two types of sorbents were prepared for extended x-ray absorption fine structure (EXAFS) analysis at SSRL, commercially-available brominated activated carbon (AC-Br) (DARCO Hg-LH, Norit Americas Inc.) and brominated activated carbon fibers (ACF-Br) (Illinois State Geological Survey and University of Illinois). The AC-Br sorbents were reacted at 30oC and 140oC and had calculated Hg concentrations of 10,817 mg/m3 and 403 mg/m3, respectively. The brominated activated carbon fiber was reacted at 140oC with a calculated total Hg concentration of 403 mg/m3. High-quality Hg LIII-edge XAS spectra of these three samples were collected at the wiggler Beam Line 7-3. Samples were slow-cooled using a modified method used by Jew et al. (2011) to detect elemental Hg, if present, in the samples.
Analysis of the x-ray absorption near-edge spectroscopy (XANES) portion of the XAS spectra indicates that all the Hg in the samples was in the Hg2+ oxidation state. The results of the XANES analysis show that the uptake of Hg onto brominated activated carbon substrates is through an oxidative process and not electrostatic adhesion of elemental Hg. The EXAFS spectra and Fourier Transform for all three samples are similar as seen in Fig. 1. Wavelet analysis (Munoz et al., 2003) of the EXAFS data indicates that a single scattering pathway is present in the data. Shell-by-shell fitting of the spectra results in a single-scattering pathway created by a Hg-Br interaction. Data fitting of the AC-Br samples reacted at both 30oC and 140oC using the Hg-Br pathway showed a Hg-Br distance of 2.55 ± 0.01 Å with a coordination number of 2.2 ± 0.1 and 2.4 ± 0.1, respectively. Though similar to the AC-Br samples, the ACF-Br sample was fit with a slightly longer Hg-Br distance of 2.58 ± 0.01 Å and a similar coordination number of 2.4 ± 0.1. There were no Hg-O or Hg-C interactions identified in the EXAFS spectra.
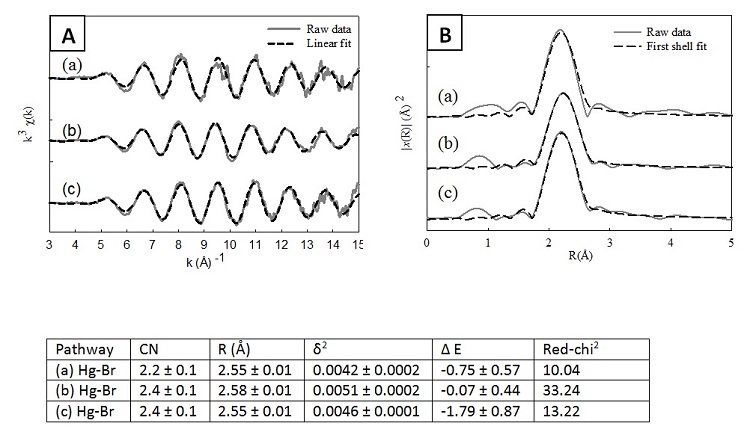
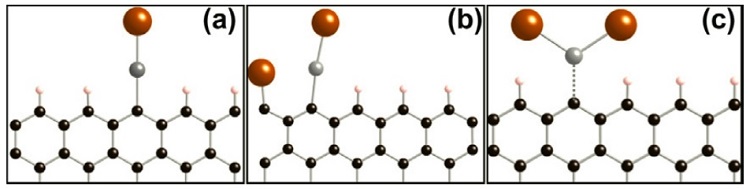
Based on the EXAFS analysis, three binding schemes are potentially occurring (Fig. 2): 1) monodentate binding of Hg to a surface carbon with an additional bond to bromide away from the graphene surface, 2) monodentate binding of Hg to a surface carbon with an additional bond to bromide away from the graphene surface and a non-binding interaction with a surface-adsorbed bromide, and 3) binding of Hg to two bromide ions with a non-binding interaction with the graphene surface. Based on the coordination numbers derived from shell-by-shell fitting, the second and third models are the most likely.
This work shows that Hg uptake to the graphene surfaces is an oxidative process. Even though a lower reaction temperature increases Hg uptake on these solids, EXAFS spectroscopy indicates that the actual binding of Hg to the surface is similar regardless of the temperature. This suggests that Hg is oxidized on the surface of the solids instead of in the gas stream since Hg0(g) volatilizes readily from surfaces at temperatures above 80oC. If the Hg were oxidized in the gas stream and settled on the activated carbon surfaces, then the total uptake of Hg on the surfaces of the AC-Br should be the same for both reaction temperatures used in this study. This work shows that by brominating activated carbon, the Hg uptake capacity is increased with Br playing a dominant role in Hg speciation and potentially Hg stability.
DFT calculations were carried out using the Vienna ab initio Simulation Package (Kresse and Hafner, 1993). Electron exchange-correlation functionals were represented with the generalized-gradient approximation (GGA), with the Perdew, Burke and Ernzerhof (PBE) model used for the nonlocal corrections (Perdew, et al., 1996). Previous work carried out by Padak and Wilcox (2010) showed that the reactivity of AC can be studied using a simplified graphene model in which a single graphene sheet mimics the AC surface. Using a 9-ring deep graphene ribbon model, electronic structure calculations were carried out to determine the most stable concentrations and configurations of H, Br and Hg atoms on the edge sites. The configuration in which Hg is directly bound to the carbon and coordinated with 2 Br atoms agrees well with the EXAFS measurements, with a prediction of 2.58 Å for each Hg-Br bond. This result indicates that Hg may be first oxidized by surface-bound Hg, leaving behind surface vacancies onto which oxidized Hg (i.e., HgBr or HgBr2) subsequently adsorbs.
- A. D. Jew, C. Kim, J. J. Rytuba, M. S. Gustin, and G. E. Brown Jr., "New Technique for Quantification of Elemental Hg in Mine Wastes and Its Implications for Mercury Evasion Into the Atmosphere", Environ. Sci. Technol. 45, 412 (2011)
- G. Kresse and J. Hafner, "Ab initio Molecular Dynamics for Liquid Metals", Phys. Rev. B 48, 13115 (1993)
- M. Munoz, P. Argoul, and F. Farges, "Continuous Cauchy Wavelet Transform Analysis of EXAFS Spectra: A Qualitative Approach", Am. Mineral. 88, 694 (2003)
- B. Padak and J. Wilcox, "Understanding Mercury Binding on Activated Carbon", Carbon 47, 2855 (2009)
- J. P. Perdew, K. Burke, and M. Ernzerhof, "Generalized Gradient Approximation Made Simple", Phys. Rev. Lett. 77, 3865 (1996)
E. Sasmaz, A. Kirchhofer, A. D. Jew, A. Saha, D. Abram, T. F. Jaramillo, and J. Wilcox, “Mercury Chemistry on Brominated Activated Carbon”, Fuel 99, 188 (2012), doi: 10.1016/j.fuel.2012.04.036.

