Arguably the most important evolutionary innovation in the history of the biosphere was the advent of oxygenic photosynthesis. Understanding how biological water-splitting evolved, however, has largely remained a theoretical exercise building on observations from biology and chemistry of modern organisms, and a variety of hypotheses have been proposed for the origin of oxygenic photosynthesis. The key to directly testing these ideas lies in reconstructing the behavior of the ancient manganese cycle from observations of sedimentary rocks.
A research team led by geobiologists from the California Institute of Technology approached this challenge by characterizing the coordination environment and redox state of manganese in a large suite of samples taken from a new set of deep scientific cores drilled in Paleoproterozoic-age strata from South Africa. It was clear from initial bulk chemical methods (e.g. ICP-MS) that these rocks contain remarkable manganese enrichments from the time interval just before atmospheric oxygen rose (~2.4 Ga, or 2.4 billion years ago). These enrichments are so substantial that it implies that Mn was oxidized in seawater prior to the rise of oxygen (and potentially in the absence of O2). Reduced manganese (Mn2+) is soluble, and can only be chemically oxidized by high potential oxidants like O2 (and O2 derived species such as O2- and H2O2). High-valent Mn-oxide phases are insoluble, causing Mn to be deposited and concentrated in marine sediments. Therefore, the goal of the study was to investigate the earliest major Mn deposits from a 2.415 Ga South African drill core record and compare the chemical insights gained from the Mn characterization performed at SSRL against independent proxies to test whether the Mn cycle was being impacted by O2 at this time in Earth history.
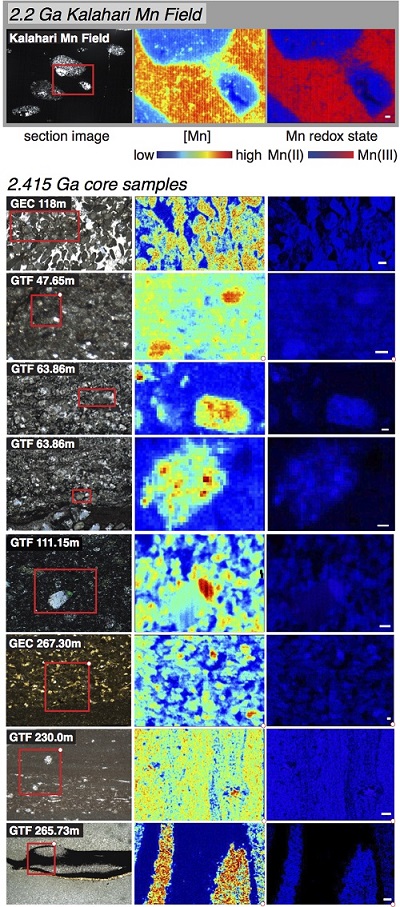
A fundamental challenge was figuring out the processes responsible for concentrating and mineralizing Mn in this ancient sedimentary basin—a task made particularly challenging because, while the deposits studied are very large, the samples contain very fine-grained mixtures of mixed-valence Mn- and Fe-bearing minerals, and understanding the phases at this microscale is critical to understanding how these minerals formed. The study utilized three beam lines at SSRL to gain a greater understanding of the 2.415 Ga manganese deposits. Beam Line 4-1 was used to characterize a large number of powder samples that had been micromilled from sedimentary textures throughout both drill cores. Extended x-ray absorption fine structure (EXAFS) spectroscopic measurements determined that in bulk, the Mn was hosted as Mn(II) in carbonate phases, either in rhodochrosite (MnCO3) or kutnohorite (Mn0.5 Ca0.5CO3). The imaging beam lines at SSRL (Beam Lines 10-2 and 2-3) allowed detailed mapping of the sedimentary textures of these deposits by examining ultrathin sample sections. These results demonstrated that the observed textures were original to the sediments as the Mn was in micron-sized authigenic grains and mm-scale nodules (diagenetic carbonates that had grown into the surrounding sediment during early diagenesis), and not in fractures or hydrothermally altered zones impacted by later fluids.
Other measurements performed included carbon isotope ratios of these carbonate crystals by gas-source mass spectrometry, which found that they did not precipitate from seawater, but instead from pore fluids influenced by the sedimentary respiration of organic matter. Though Mn was originally concentrated in the sediments as insoluble oxides, no sign of the early oxides could be found even using the 2µm microprobe beam at Beam Line 2-3 (this contrasts with microprobe results from younger deposits such as the 2.2 Ga Kalahari Mn field from South Africa). Instead, all of the Mn oxides were converted to Mn carbonates by metal-reducing microbes in a process known today from a variety of bacteria. This metabolism produces alkalinity and dissolves inorganic carbon promoting the precipitation of carbonate phases. The observation that all of the Mn was reduced during early diagenesis is intriguing as Mn-oxide reduction only occurs when no oxygen is present—suggesting that these Mn deposits were not formed by O2 but rather a different high-potential oxidant.
When combined with independent geological and geochemical observations, the x-ray data allowed the development of a high-level working hypothesis for the processes of Mn mineralization and petrogenesis in these sedimentary deposits. The x-ray spectroscopy and imaging work of the Mn enrichments at 2.415 Ga was paired with two independent proxies for oxygen: multiple sulfur isotopes and the presence or absence of oxygen-sensitive detrital grains such as pyrite and uraninite. At first, the sulfur isotopes of pyrite appeared to record a major transition since the systematics of the multiple sulfur isotopes (mass-independent fractionations in the minor 33S and 36S isotopes and the magnitude of mass-dependent fractionations of 34S) seemed to completely shift during the core deposition. However, close examination of the textures of the pyrite allowed the separation of the true S signal held in original pyrite from sulfur isotopes hosted by secondary pyrite in veins, cracks, rims, and other diagnostic textures for minerals derived from later altering fluids. With this screening, it was found that all of the original pyrite recorded S signals of an oxygen-free atmosphere. Additionally, the sandstone intervals of the cores were examined to see if there were known oxygen-sensitive grains like pyrite and uraninite along with the quartz sand grain—and indeed, abundant detrital pyrite grains were found.
These two proxies revealed that the atmosphere was essentially devoid of oxygen. How could there have been Mn oxide production (but not stabilization) without oxygen present? A class of hypotheses developed from biochemistry and comparative biology suggest that a transitional manganese-oxidizing photosystem (producing Mn oxides as a product) existed before water-oxidizing photosynthesis (producing oxygen as a product). The 2.415 Ga Mn enrichments provide direct evidence from the geological record of these biological theories.
J. E. Johnson, S. M. Webb, K. Thomas, S. Ono, J. L. Kirschvink, and W. W. Fischer, “Manganese-oxidizing Photosynthesis before the Rise of Cyanobacteria”, Proc. Natl. Acad. Sci. USA 110, 11238 (2013); doi: 10.1073/pnas.1305530110

