Li-ion batteries are regarded as key devices in the effort to develop efficient chemical energy storage from sustainable energy sources. Further development and maximization of their performance are essential, but these must be achieved using safe, cheap, abundant and environmentally friendly materials. For optimized performance, understanding of fundamental mechanisms of diffusion and phase transformation is mandatory. Practical electrodes for Li-ion batteries have hierarchical architectures with small particles of active material distributed in a matrix of binder and conductive carbon. Using the recently highlighted mesoscale technique [1] of full-field transmission x-ray microscopy coupled with x-ray absorption near-edge spectroscopy (FF TXM-XANES), the chemical state of the active metal within electrodes can be used to build maps of material utilization and establish pathways for the electrochemical reaction at the single particle level. In this study supported by the Northeastern Center for Chemical Energy Storage, an Energy Frontier Research Center funded by the U.S. Department of Energy, scientists from Lawrence Berkeley National Laboratory and SLAC designed methodologies to visualize the chemical phase transformations occurring in single crystals of electrode materials of interest in Li-ion technologies.
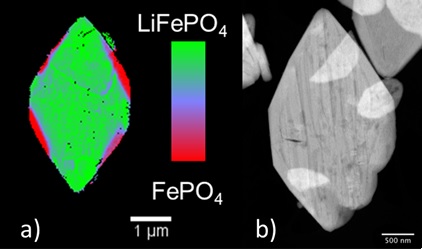
Although LiFePO4 is widely considered as a cathode material that has achieved technological maturity, the fundamentals of the phase transformation remain poorly understood, with a large variety of conflicting mechanisms being proposed [2,3]. Maps of the distribution of species involved in the transformation (LiFePO4 and FePO4) were produced at high chemical and spatial (30 nm) resolution for partially delithiated, micron-sized LiFePO4 plate-like crystals using FF TXM-XANES at SSRL Beam Line 6-2. These maps were compared to images containing morphological information collected by scanning transmission electron microscopy (STEM), leading to a complete picture of the interplay between microstructure and redox reactions at the mesoscale (Figure 1). Delithiation was found to be more favorable at edge locations than in the center of the crystals. When extensive delithiation was observed in the center, evidence was found of the formation of elongated domains in a striped pattern that was significantly different from the edges. Comparison with STEM images revealed that this complex behavior resulted from a combination of kinetic and thermodynamic limitations, the former being determined by the presence of macroscopic defects in the crystals. Stresses accompanying the delithiation process led to the cracking of the particles due to the buildup of strain at the phase boundaries.
The results of this study stress the role of microstructure as a kinetic factor during the redox transformation of a particle between initial and final state, especially when defects are considered. It provides clues to the design of electrode materials with enhanced utilization and durability. It also constitutes a highly representative example of the capabilities of TXM-XANES to study processes relevant to energy applications.
- Department of Energy (2012) Report, “From Quanta to the Continuum: Opportunities for Mesoscale Science”, p. 25
- G. Chen, X. Song and T. J. Richardson, Electrochem. Solid-State Lett. 9, A295 (2006)
- C. Delmas, M. Maccario, L. Croguennec, F. Le Cras and F. Weill, Nature Mater. 7, 665 (2008)
U. Boesenberg, F. Meirer, Y. Liu, A. K. Shukla, R. Dell’Anna, T. Tyliszczak, G. Chen, J. C. Andrews, T. J. Richardson, R. M. Kostecki and J. Cabana, " Mesoscale Phase Distribution in Single Particles of LiFePO4 following Lithium Deintercalation", Chem. Mater. 25, 1664 (2013) doi. 10.1021/cm400106k.

