Eukaryotic Cys-loop receptors control fast synaptic transmission and are important targets for various therapeutics, including general anesthetics. Despite their biological importance, high-resolution structures for Cys-loop receptors remain scarce because of many technical challenges. Prokaryotic pentameric ligand-gated ion channels (pLGICs) from Erwinia chrysanthemi (ELIC) (Hilf and Dutzler 2008) and Gloebacter violaceus (GLIC) (Bocquet, Nury et al. 2009; Hilf and Dutzler 2009) are prototypes of Cys-loop receptors. The resemblance of their pharmacological properties to Cys-loop receptors and their amenability to crystal structure determination make ELIC and GLIC ideally suited for structural elucidation of allosteric activation and modulation of pLGICs. Specifically, in this study two lines of investigation were carried out: the structural underpinning of the pLGIC activation process and the structural basis of anesthetic modulation of pLGICs.
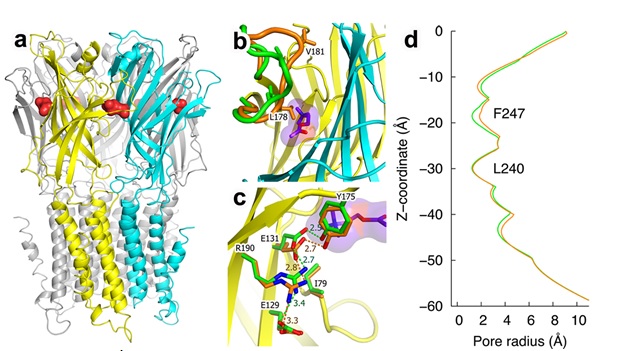
To understand the pLGIC activation process, ELIC was co-crystallized with acetylcholine (ACh), an endogenous agonist for nicotinic acetylcholine receptors (nAChRs) in the Cys-loop family. The 2.9-Å resolution structure obtained from data collected at SSRL’s microfocus BL12-2 shows ACh binding to the orthosteric ligand site in the extracellular (EC) domain of ELIC (Fig. 1a). ACh induced conformational rearrangements in the EC domain (Fig. 1b), resembling those observed in the agonist-bound acetylcholine binding proteins AChBPs (Celie, van Rossum-Fikkert et al. 2004) and α7nAChR-AChBP chimera (Li, Huang et al. 2011). A comparison of the ELIC structures with and without a bound ACh highlights the importance of cation-π and other electrostatic interactions in the ligand binding and channel activation process. Moreover, the structural comparison revealed the signal propagation underlying ELIC function (Fig. 1c). However, functional measurements indicated that ACh acts as a competitive antagonist instead of an agonist for ELIC. Consistent with the functional result, the crystal structure shows an enlarged pore size at the hydrophobic restriction region of ELIC upon ACh binding, but the enlargement is not great enough to open the channel (Fig. 1d). It appears that ACh binding brings ELIC to the verge of activation. Indeed, a simple substitution from –CH3 to –H in the ACh’s choline group was sufficient to convert the ligand from a competitive antagonist into an agonist. Because co-crystallization of ELIC with a high-concentration of agonist is likely to produce ELIC crystals in a desensitized state, the crystal structure of the ACh-ELIC complex on the verge of activation offers a useful template for delineating structure-function relationships of Cys-loop receptors in action. This high-resolution picture of ACh binding provides insights into the structural underpinning of agonism versus competitive antagonism instrumental for designing new therapeutic drugs with optimized atomic interactions that can potentially suppress or enhance certain conformational states, thereby modulating the functions of Cys-loop receptors.
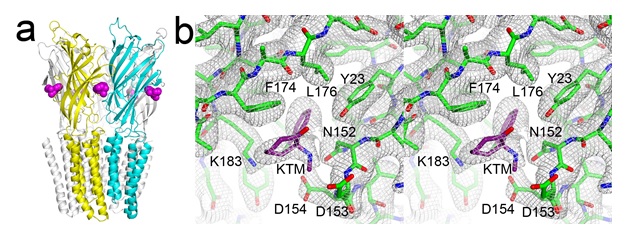
Clinical application of general anesthetics is indispensable in modern medicine, but the molecular mechanisms of general anesthesia remain unclear. Cys-loop receptors are targets of general anesthetics. It remains a formative challenge to pinpoint where and how anesthetics act on these channels to modulate their functions. Ketamine is widely used for the induction and maintenance of general anesthesia. Although ketamine is commonly known as a dissociative anesthetic acting as a noncompetitive antagonist on the N-methyl-D-aspartate (NMDA) receptor, it is also a potent inhibitor of neuronal nAChRs. The sites of action for ketamine at these receptors have not been identified previously. This study shows that ketamine inhibits GLIC with a half-maximal inhibition concentration comparable to that of neuronal nAChRs. The crystal structure of the GLIC-ketamine complex with a resolution of 2.99-Å (again with data collected from SSRL Beam Line 12-2) reveals a novel anesthetic binding site in a preexisting amphipathic cavity in the EC domain of GLIC (Fig. 2). Functional relevance of this site is evident in electrophysiology measurements of mutations in this region, and the effects of subsequent chemical labeling to mimic anesthetic binding. The study provides compelling evidence for a mechanism of the allosteric inhibition of pLGICs by anesthetics. The study also suggests that the anesthetic action of ketamine is more than just as an antagonist for the NMDA receptor.
- N. Bocquet, H. Nury, et al., "X-ray Structure of a Pentameric Ligand-gated Ion Channel in an Apparently Open Conformation", Nature 457, 111 (2009)
- P. H. Celie, S. E. van Rossum-Fikkert, et al., "Nicotine and Carbamylcholine Binding to Nicotinic Acetylcholine Receptors as Studied in AChBP Crystal Structures", Neuron 41, 907 (2004)
- R. J. Hilf and R. Dutzler, "X-ray Structure of a Prokaryotic Pentameric Ligand-gated Ion Channel", Nature 452, 375 (2008)
- R. J. Hilf and R. Dutzler, "Structure of a Potentially Open State of a Proton-activated Pentameric Ligand-gated Ion Channel", Nature 457, 115 (2009)
- S. X. Li, S. Huang, et al., "Ligand-binding Domain of an Alpha(7)-Nicotinic Receptor Chimera and Its Complex with Agonist." Nat. Neurosci. 14, 1253 (2011)
J. Pan, Q. Chen, D. Willenbring, K. Yoshida, T. Tillman, O. B. Kashlan, A. Cohen, X.-P. Kong, Y Xu, and P. Tang, “Structure of ELIC Co-crystallized with Its Competitive Antagonist Acetylcholine”, Nat. Commun. 3, 714 (2012) doi: 10.1038/ncomms1703
J. Pan, Q. Chen, D. Willenbring, D. Mowrey, X.-P. Kong, A. Cohen, C. B. Divito, Y. Xu and P. Tang, "Structure of the Pentameric Ligand-Gated Ion Channel GLIC Bound with Anesthetic Ketamine", Structure 20, 1463 (2012) doi: 10.1016/j.str.2012.08.009

