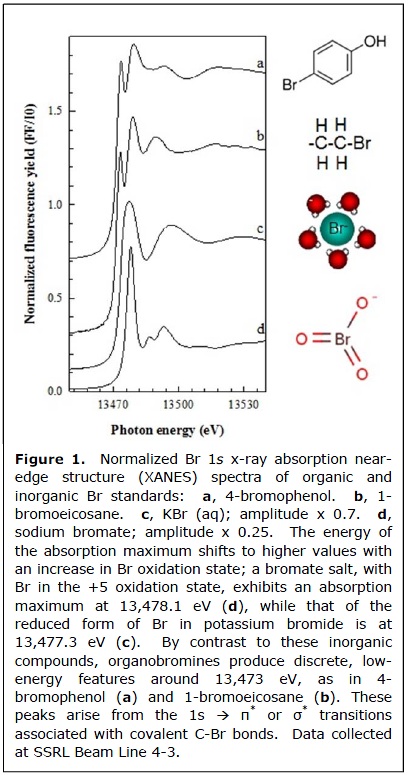
For many years, the chemistry of bromine in the environment has been presumed altogether unreactive. In seawater, bromine has long been classified as a conservative element and thought to exist in the form of inorganic bromide. In terrestrial soils, bromide is believed to be so unreactive that it is routinely used as a hydrological tracer.
There were signs, however, that the redox behavior of bromine might be more complex than imagined. Total bromine concentrations were found to be strongly correlated with organic carbon content in marine sediments.1 Meanwhile, more and more organobromine natural products were being discovered and catalogued.2 Since organobromines tend to be toxic, it seemed logical that organisms might produce them for chemical defense.
Recent x-ray absorption spectroscopic (XAS) studies performed at SSRL Beam Lines 2-3 and 4-3, as well as at the ALS and NSLS, have revealed that the association between bromine and organic carbon is attributable to covalent carbon-bromine bonds. Differences in the near-edge spectral features of organic vs. inorganic forms of bromine (Fig. 1) make XAS a useful tool to distinguish between them in heterogeneous environmental samples. Br XAS experiments conducted by Leri et al. revealed organobromine to be the dominant form of bromine in sedimentary organic matter in samples from various locations around the globe. In this study, published in Global Biogeochemical Cycles in 2010, absolute organobromine concentrations in sediment samples were measured by combining XAS measurements of bromine speciation with XRF measurements of bromine concentration with x-ray fluorescence (XRF). The resulting data showed that organobromine is strongly correlated with total organic carbon, and both parameters decrease with sediment depth in most locations. Associations between organobromine, organic carbon, and metals like iron became strikingly apparent in x-ray microscope maps showing the distributions of these elements in undisturbed sediment core sections (Fig. 2). The researchers also found high levels of organobromine in sinking particulates from the water column, linking organobromine in sediments with biological processes in the euphotic zone of seawater.
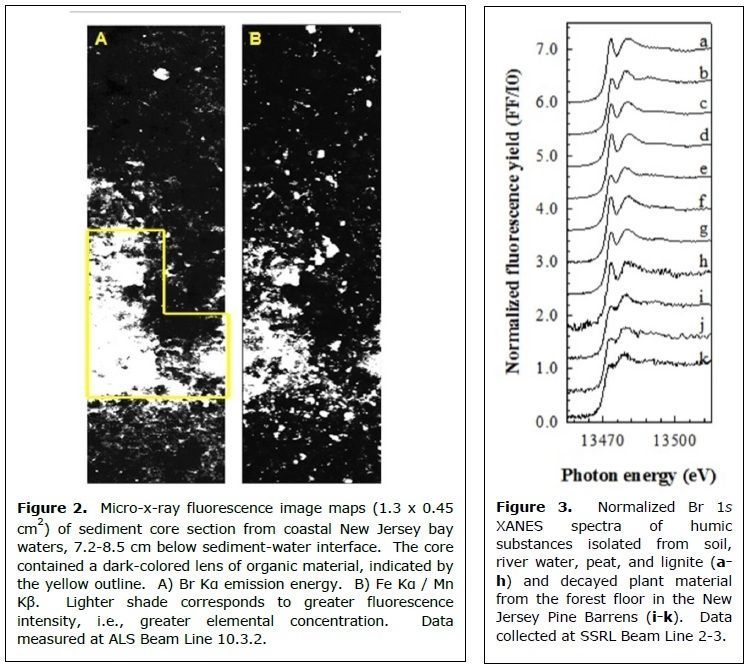
Leri and Myneni recently discovered similar trends in the terrestrial environment, looking at decaying plant material on the forest floor. This study, published in Geochimica et Cosmochimica Acta earlier this year, demonstrated that all bromine present in humic substances and soil organic matter is covalently bonded to carbon (Fig. 3). Common halogenating enzymes were shown to readily brominate healthy plant material, illuminating an environmentally feasible mechanism of organobromine production. The results suggest that inorganic bromide functions as a limiting reagent in the natural bromination of soil organic matter.
These XAS studies have dramatically changed the view of bromine in the environment. Where this element was once believed to exist as an unreactive inorganic species, it is now clear that it participates in dynamic biogeochemical cycling between inorganic and organic forms. The large-scale bromination of natural organic matter may have far-reaching consequences for the preservation and degradation of organic carbon in soils and sediments. Although organobromine might be expected to function as a comparatively recalcitrant form of organic carbon, the ratios of organobromine to organic carbon decrease with sediment depth in several locations. This raises the counter-intuitive possibility that brominated organic matter is preferentially degraded during diagenesis. The natural bromine cycling identified in these studies may also have implications for the fate of brominated environmental pollutants, such as polybrominated diphenyl ether (PBDE) flame retardants. It furthermore calls into question the use of bromide as a hydrological tracer.
This investigation was funded by the U.S. Department of Energy, Office of Basic Energy Sciences (DOE-BES) Chemical and Geosciences Programs, the National Science Foundation (NSF) Chemical Sciences Program, and an NSF Graduate Research Fellowship (ACL).
- L. Mayer, S. Macko, W. Mook, and S. Murray (1981), Organic Geochemistry, 3, 37-42.
- G. Gribble (1999), Chemical Society Reviews, 28, 335-346.
A. Leri, J. Hakala, M. Marcus, A. Lanzirotti, C. Reddy, and S. Myneni (2010), Natural organobromine in marine sediments: New evidence of biogeochemical Br cycling, Global Biogeochemical Cycles, 24, GB4017.
A. Leri and S. Myneni (2012). Natural organobromine in terrestrial ecosystems, Geochimica et Cosmochimica Acta, 77, 1-10.

