 Wnt proteins engage an array of receptors and inhibitors to precisely regulate crucial processes during embryonic development and tissue homeostasis and repair in the adult, and deregulated Wnt signaling is observed in many types of cancers and degenerative diseases. The seven-pass transmembrane receptors Frizzled (Fz) are essential for nearly all Wnt signaling, and bind to Wnts primarily through their cysteine-rich ectodomains (CRD). Mammals encode 19 Wnt proteins and 10 Fz receptors, and it is unclear if mono-specific Wnt – Fz pairs are responsible for certain biological functions and diseases. However, it has been shown that Wnt proteins can engage multiple Fz, and Fz receptors can respond to multiple Wnts. This cross-reactivity has complicated the unambiguous matching of interacting Wnt – Fz pairs, and their attribution to certain biological functions and disease. In the July 6 issue of Science, we present the first crystal structure of a Wnt protein bound to the extracellular domain of Fz receptor, which sheds light on the molecular basis of Wnt – receptor specificity and receptor activation.
Wnt proteins engage an array of receptors and inhibitors to precisely regulate crucial processes during embryonic development and tissue homeostasis and repair in the adult, and deregulated Wnt signaling is observed in many types of cancers and degenerative diseases. The seven-pass transmembrane receptors Frizzled (Fz) are essential for nearly all Wnt signaling, and bind to Wnts primarily through their cysteine-rich ectodomains (CRD). Mammals encode 19 Wnt proteins and 10 Fz receptors, and it is unclear if mono-specific Wnt – Fz pairs are responsible for certain biological functions and diseases. However, it has been shown that Wnt proteins can engage multiple Fz, and Fz receptors can respond to multiple Wnts. This cross-reactivity has complicated the unambiguous matching of interacting Wnt – Fz pairs, and their attribution to certain biological functions and disease. In the July 6 issue of Science, we present the first crystal structure of a Wnt protein bound to the extracellular domain of Fz receptor, which sheds light on the molecular basis of Wnt – receptor specificity and receptor activation.
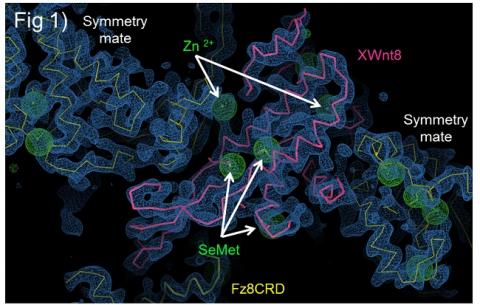
Wnt proteins are lipid-modified, complicating the expression, purification and crystallization of active Wnts. We overcame these problems by co-expressing XWnt8 and Fz8-CRD in Drosophila S2 cells, and purifying the soluble complex in the absence of detergent. This complex yielded diffraction quality crystals. X-ray diffraction data were collected using Beam Line 11-1 at the Stanford Synchrotron Radiation Lightsource. To obtain phases information, we prepared selenomethinone (SeMet)-derivatised XWnt8-Fz8CRD crystals and experimental phases were determined by single isomorphous replacement with anomalous scattering (SIRAS). The initial experimental map (Figure 1) showed very clear electron density into which the fully modified XWnt8-Fz8CRD complex could be build. The SeMet sites, bulky side chain residues and disulfide bonds were used to trace and to confirm the register of the polypeptide chain.
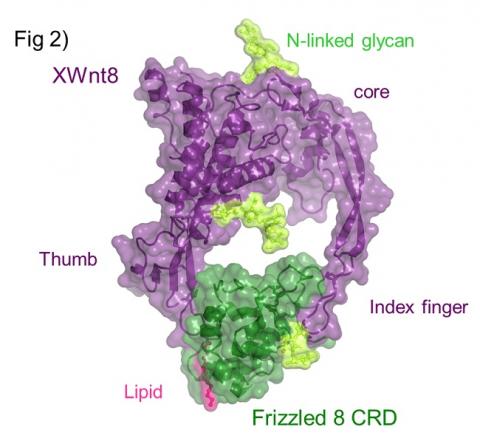
XWnt8 has an unusual two-domain structure (Figure 2), and each domain extends a beta-strand or ‘finger’, which ‘grasps’ the Fz8-CRD on opposite faces. Site 1 interaction is primarily mediated by a palmitoleic acid covalently attached to a conserved Serine at the tip of ‘thumb’, which binds within a conserved hydrophobic groove in Fz8-CRD. In site 2, the tip of a long beta-stand or 'index finger' containing several hydrophobic residues binds a hydrophobic depression on Fz8-CRD. While residues on XWnt8 interface are conserved among other Wnts, residues on the Fz8-CRD interface are more variable. Binding studies demonstrated that the C-terminal domain of XWnt8 principally consisting of the ‘index finger’, binds Fz-CRDs with comparable specificity as full-length XWnt8. These results suggest that Fz discrimination is primarily achieved by the site 2 interface. Thus, we conclude that the high conservation of residues in both interface 1 and 2 is consistent with Wnt – Fz cross-reactivity rather than mono-specificity, and the observed affinity differences between Wnt - Fz pairs could potentially tune Wnt signaling outcome.
The crystal structure of the XWnt8 - Fz8-CRD complex presents the first molecular snapshot of Wnt signaling activation, and provides a conceptual and technical framework for future research elucidating the molecular basis of Wnt signaling activation and inhibition and Wnt signaling specificity.
Structural Basis of Wnt Recognition by Frizzled. Claudia Y. Janda, Deepa Waghray, Aron M. Levin, Christoph Thomas, and K. Christopher Garcia. Science. 337, 59-64, 2012. doi: 10.1126/science.1222879