Changing the electronic structure of a metal in order to “tune” its affinity to catalytic reaction intermediates is a key element in catalyst design. Tailor-made catalysts with a carefully adjusted ratio of two or more different alloy components are particularly needed in fuel cells, which could efficiently power electric vehicles – without the range limitations of current batteries. Both convert chemically stored energy into electricity, but in batteries this storage is limited to the electrode materials themselves, while fuel cells draw hydrogen or methanol from a tank and oxygen from air.
While the overall chemical reaction in a fuel cell is the same as in a combustion engine, its energy conversion is much more efficient since the reaction is separated in oxidation of the fuel at the anode, and the oxygen reduction reaction (ORR) at the cathode; the reaction free enthalpy is therefore directly converted into electricity. However, especially for the ORR an extremely careful design of catalyst materials is needed in order to meet two requirements: high catalytic activity to minimize costly use of its active component, Pt, and, at the same time, high stability to prevent catalyst degradation during long-term operation in a corrosive environment.
Bimetallic ORR catalysts exhibit expanded or compressed Pt–Pt distances (strain effect) as well as orbital interactions between Pt and the other metal (ligand effect); both effects change the chemisorption energy of atomic oxygen. The catalytic ORR activity correlates with the latter in a “volcano” relationship and becomes maximal when O is bound ~0.2 eV more weakly than on Pt(111).1
Unfortunately, most often attempts to meet both requirements of high ORR activity and catalyst stability have failed, since the most active catalysts, e.g., Pt3Ni,2 are not stable enough and, conversely, the most stable catalysts have only poor ORR activity.
Recently, SIMES and SUNCAT researchers joined their efforts and examined the “tuning” of a bimetallic fuel cell catalyst using well-defined model systems, consisting of ultrathin Pt layers grown on a single-crystal Rh(111) substrate. They discovered that the discrepancy between catalyst activity and stability could be overcome if, in addition to its elemental composition, its nanostructure is made a design criterion.
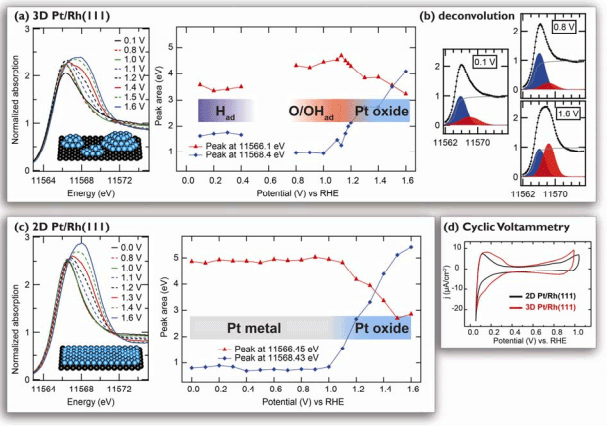
The SIMES team, led by Associate Staff Scientist Daniel Friebel, probed the nanostructure of Pt/Rh(111) model catalysts using the grazing incidence x-ray absorption fine structure (GI-XAFS) technique at SSRL Beam Line 11-2. It was found that a small Pt amount that corresponds to a single atomic layer can be grown on the Rh substrate in two different ways, resulting either in three-dimensional Pt islands (3D Pt/Rh(111)) or in a uniform two-dimensional Pt monolayer (2D Pt/Rh(111)). Both structures were then examined in 0.01 M HClO4 electrolyte at various electrochemical potentials with x-ray absorption spectroscopy (XAS) using the high energy resolution fluorescence detection (HERFD) mode at SSRL Beam Line 6-2. Uwe Bergmann supported this experiment with a unique setup of Bragg analyzer crystals for the HERFD technique, which allows for the in situ detection of chemisorbed oxygen species (O/OHad) whose spectral signatures would be too subtle to be resolved with conventional XAS. Due to their different nanostructures, the two samples showed markedly different behavior: 3D Pt/Rh(111) exhibited a much greater affinity to O/OHad than the 2D Pt/Rh(111), with O/OHad almost completely absent on the latter (Fig. 1).

SUNCAT graduate student Venkat Viswanathan performed DFT calculations on various Oad–Pt/Rh(111) model structures and found a simple correlation between oxygen chemisorption energy and the local metallic coordination environment. The strain effect due to the ~3% compression of the Pt layer to the smaller Rh lattice constant and the ligand effect both cause a significant weakening of the Pt–Oad that places 2D Pt/Rh(111) on the far opposite side of the fuel cell volcano as seen from Pt(111). On the surface of 3D Pt islands, the local Pt layer thickness is greater than one monolayer around most adsorption sites and, in addition, adsorption sites with under-coordinated Pt atoms exist at edges and corners. Therefore, strain and ligand effects are partly compensated and the O adsorption energy is shifted back towards that of Pt(111). Most notably, site-specific adsorption energies were found to be wide-spread in energy such that some individual sites could even reach the volcano maximum. Based on a careful analysis in which sites that are inaccessible due to Oad–Oad repulsion were excluded (Fig. 2), the researchers predict that sites adjacent to “B-edges” of Pt bilayer islands are most active, and that a tailored Pt-Rh nanostructure with maximized occurrence of the most active sites could have up to 5-fold ORR activity enhancement over pure Pt (Fig. 2b). Moreover, due to high cohesion energies such a structure is expected to be much more stable against catalyst degradation than Pt/Ni and Pt/Co catalysts2,3 with comparable activity; thus, both requirements of high activity and stability could be fulfilled at the same time.
This work is supported by the Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under contract DE-AC02-76SF00515 and Division of Chemical Sciences through the SUNCAT Center for Interface Science and Catalysis. This research was partly carried out at the Stanford Synchrotron Radiation Lightsource, a National User Facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences. D.F. is grateful to the Alexander von Humboldt Foundation for a Feodor Lynen Fellowship. The Center for Atomic-Scale Materials Design is funded by the Lundbeck Foundation. We acknowledge support from the Danish Center for Scientific Computing. We thank Uwe Bergmann for support and discussions of the HERFD XAS experiments, and John Bargar for providing the GI-EXAFS setup.
(1) Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J. K. Nature Chem. 2009, 1, 552–556.
(2) Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G. F.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Science 2007, 315, 493–497.
(3) Stamenkovic, V.; Mun, B. S.; Mayrhofer, K. J. J.; Ross, P. N.; Markovic, N. M.; Rossmeisl, J.; Greeley, J.; Nørskov, J. K. Angew. Chem. Int. Ed. 2006, 45, 2897–2901.
(4) Zhang, J. L.; Vukmirovic, M. B.; Xu, Y.; Mavrikakis, M.; Adzic, R. R. Angew. Chem. Int. Ed. 2005, 44, 2132–2135.
D. Friebel, V. Viswanathan, D. J. Miller, T. Anniyev, H. Ogasawara, A. H. Larsen, C. P. O’Grady, J. K. Nørskov, and A. Nilsson, "Balance of Nanostructure and Bimetallic Interactions in Pt Model Fuel Cell Catalysts: In Situ XAS and DFT Study", J. Am. Chem. Soc.134, 9664 (2012)




