Desensitization of Phototransduction
In animals subjected to dark-adaptation, the phototransduction cascade of rod photoreceptors is extremely sensitive and capable of responding to a single photon. Signalling in response to light is initiated by the receptor, rhodopsin, and mediated by the G protein, transducin, consisting of three subunits--α, β and γ. The transducin α-subunit contains the guanine nucleotide exchange site and is membrane-associated via N-terminal lipidation at Gly-2 carrying a side chain C12:0, C14:1, C14:2 or C14:0. Transducin β- and γ-subunits, forming a heterodimer, are membrane-bound via the C-terminal farnesylation of Tγ. A fascinating observation is that transducin translocates to the rod inner segment in-bulk during intense light exposure. In the translocation process, transducin is displaced from disc membranes which has the effect of desensitizing the signalling cascade, which constitutes an important mechanism for light adaptation. Under intense light, TαGTP and Tβγ translocate individually to the inner segment (IS) by diffusion with a t1/2 of ~3 minutes. Both Tα and Tβγ return to the outer segments (OS) slowly in hours during prolonged dark-adaptation. A key component for the return of Tα to the OS is the acyl-binding protein, Uncoordinated 119 (UNC119). UNC119 interacts with transducin via the acyl side chain attached to G2. To test for its physiological function, we exposed WT and Unc119–/– mice to intense light for 60 min followed by prolonged dark-adaptation (0-24 hours). Return of Tα is nearly complete in WT photoreceptors after three hours of dark-adaptation, yet a significant amount of Tα remains associated with Unc119–/– inner segment membranes suggesting that return to the OS is mediated by UNC119.
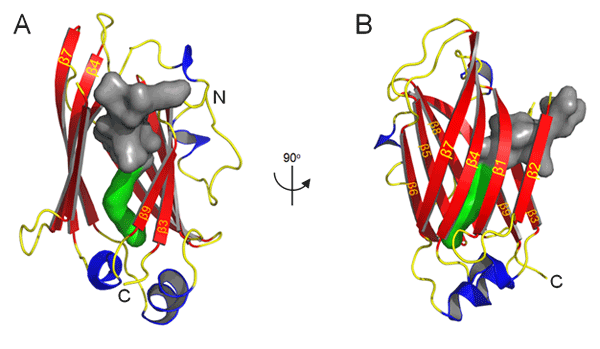
Co-crystal structure of a lipidated Tα N-terminal peptide with UNC119.
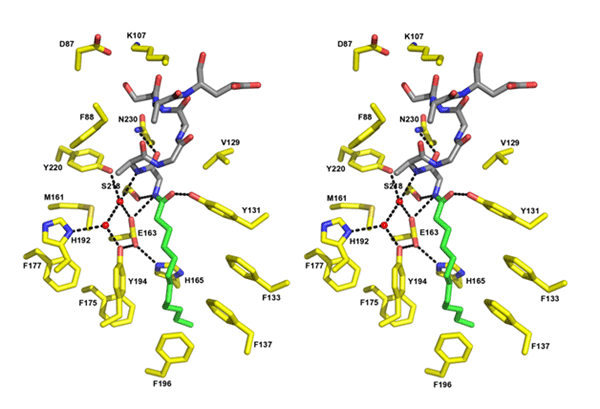
Isothermal titration calorimetry (ITC) was the method used to determine the binding constant of acylated N-terminal G protein α-subunit peptides with UNC119. ITC measures the binding enthalpy of two reactants, thus enabling the resolution of two or more binding sites. ITC experiments showed that lauroyl- and myristoyl-GAGASAEEKH each exhibited tight binding at a single site with K<sub>D</sub>s of ~0.54 μM ± 0.28 μM and 0.22 μM ± 0.14 μM, respectively. Crystal structure at 1.95Å resolution reveals that UNC119 adopts an immunoglobulin-like β-sandwich fold. Our structural and biochemical results suggest that the lipid is inserted into the hydrophobic cavity centered within the β-sandwich. Co-crystal structure of UNC119 with the lauroylated Tα peptide (2.0 Å) shows that the pocket easily accommodates a lauroyl (C12:0) moiety in a very specific fashion as each molecule in the asymmetric unit of the crystal contains a similarly bound ligand. The cavity is lined by hydrophobic residues (predominantly Phe and Tyr) that mediate the interaction with the lauroylated Ta peptide primarily by Van der Waals forces.
Remarkably, the entrances to the lipid-binding sites of PrBP/δ and RhoGDI, two known lipid binding proteins that exhibit similar immunoglobulin-like β-sandwich folds, do not exist in UNC119 but instead are found on the opposite edge of the β-sandwich.
The lauroyl group and Tα peptide residues 1-6 are buried deeply in UNC119's hydrophobic pocket, whereas peptide residues 7-10 make only peripheral contact with UNC119 and are poorly ordered. In general, the pocket residues form a surface that is highly complementary to the ligand shape which accounts for the high degree of conservation between the N-terminal residues of Tα and contacting residues of UNC119. These interactions provide specific recognition of N-terminal groups that define the location of the peptide-acyl junction within the UNC119 cavity, thereby establishing the acyl chain length that can be accommodated.
Work on UNC119 was supported by National Institute of Health grants EY08123, EY019298 (WB), EY014800-039003 (NEI core grant), EY10848 (GI), by the Howard Hughes Medical Institute (EJ) and by NIH Grant NS034307 (EJ), by a grant from the Protein Structure Initiative of the National Institutes of Health (U54 GM074958), by the University of Utah Macromolecule Crystallography Core Facility, by a Center Grant of the Foundation Fighting Blindness, Inc., to the University of Utah, and unrestricted grants to the Departments of Ophthalmology at the University of Utah from Research to Prevent Blindness (RPB; New York).
Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, Inana G, Whitby FG, Hill CP, Jorgensen EM, Tong L, and Baehr W (2011) UNC119 regulates G protein trafficking in sensory neurons. Nature Neuroscience, 14(7):874-880.
Eyal Vardy & Bryan L Roth. Vision and olfaction say UNC-le to G proteins. News and Views in Nature Neuroscience 14(7), 805-6.

