
Environmentally induced adaptations exist in most organisms. Adaptive changes in form—common to load bearing joints within humans' musculoskeletal, and oral and craniofacial systems—are a result of functional demands. Adaptation in organs, on the other hand, is due to the continuous interplay between form and function, which is orchestrated by physico-chemical changes in tissues and at the biological interfaces of the organ. As such, mineralization in these musculoskeletal and other systems is a load-mediated adaptive process necessary to accommodate functional and/or therapeutic forces (including orthopedic fixation devices and orthodontic braces). This is because forces generate strain fields in tissue matrices and at the soft-hard and hard-hard biological tissue interfaces of an organ; cell-cell and cell-matrix interactions, through integrin-based coupling, sense these tension- and compression- based strain fields. Function-based mechanical energy is then transduced to chemical energy through cells' biochemical expression. The type and concentration of biochemicals can cause mineral formation or resorption, which, within an organ's tension-compression based matrices, alters the overall morphology and function of a load bearing joint.
Throughout development and growth, mineralized tissues within joints are composite-like materials with a heterogeneous distribution of organic and inorganic components. The inorganic component is predominantly composed of apatite glued together by organic globular and fibrillar proteins. However, the amount of mineral and the ratio of mineral to organic are also regulated by the functional demands on load bearing tissues. One mineral common to mineralized tissues is apatite, whose basic elements include calcium and phosphorus.
Morphological changes in this study included stratified growth of bone into the functional space of the joint, impairing joint motion to loads. The joint of interest in this study is the dentoalveolar complex in which the cementum (40-50% mineral) of a tooth is attached to the alveolar bone (50-60%) of the jaw with a soft fibrous tissue, the periodontal ligament (PDL). Under normal conditions a functional space of 150-380 microns is maintained with a decrease in space due to increase in age of the human. In this study, narrowed PDL-spaces were identified in a seemingly normal human bone-PDL-cementum complex. Narrowing was caused by several long stratified layers and/or protrusions of bone into the PDL-space. The adapted regions of bone near the non-mineralized 5-50 µm PDL were identified as chemically and mechanically heterogeneous using the SSRL Beam Line 2-3 x-ray microprobe, complemented with AFM-based nanoindentation techniques at the University of California, San Francisco.
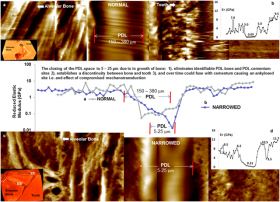
Adapted biomineralized regions were found to be higher in calcium and phosphorus (Fig. 1) with increased elastic modulus (Fig. 2) values when compared to bone and cementum within a normal functional PDL-space (Figs. 1 and 2). More importantly, narrowed PDL-spaces demonstrated discontinuities (steeper gradients) in chemical and modulus profiles, compared to the naturally graded interfaces of the bone-PDL and cementum-PDL entheses within a functional space. These significant changes in the PDL-space due to the ingrowth of bone are identified as modeling related events and are a response to functional demands. These adapted sites can act as constriction sites, impeding natural range of tooth motion within the bony socket. Constriction sites over time can become into new load bearing sites and could eventually cause direct local fusion (ankylosis) of bone with cementum.
Jonathan M. Hurng, Michael P. Kurylo, Grayson W. Marshall, Samuel M. Webb, Mark I. Ryder, and Sunita P. Ho "Discontinuities in the Human Bone-PDL-Cementum Complex" Biomaterials 32 7106-7117 (2011).




