Nitrogen is key ingredient of life, being a necessary element for all amino acids, nucleic acids and many vitamins. Despite the abundance of nitrogen in the air in our biosphere, sources of bio-available nitrogen is a limiting factor. The gaseous dinitrogen (N2) molecule is chemically inert, rendering this source unavailable for most organisms. Symbiotic bacteria in legume nodules as well as certain free living soil bacteria (among them an organism called Azotobacter vinelandii) have evolved the ability to convert N2 to ammonia (a process known as “nitrogen fixation”), thus making this vast pool of atmospheric nitrogen accessible to plants, animals and other organisms.
In the early 1900s Haber and Bosch developed an industrial process to convert N2 into forms of nitrogen that could be used by growing organisms. The process utilizes high pressure and high temperatures, and consumes a large amount of energy. Nevertheless, it is commercially very important and today provides about 50% of the “fixed” nitrogen products world-wide. The nitrogenase-enabled process, on the other hand, proceeds at ambient temperature and pressure. During the last 50 years researchers from different disciplines have tried to elucidate the mechanism of nitrogenase (also called NifDK after the names of the genes coding for its subunits), the key enzyme involved in this conversion.
The majority of biochemical and structural work has been done investigating the enzymes fromAzotobacter vinelandii(Av). Milestones were the initial establishment of the active site metal cluster structure through x-ray absorption spectroscopy in the 1970’s, elucidation of the protein structure in 1992, and the determination of its atomic resolution structure in 2002. Despite exceptionally detailed knowledge of the structure of the protein and the inorganic co-factor (called FeMoco; a 7Fe-1Mo-9S-1X-homocitrate cluster, where X is a light atom) involved in the nitrogen reduction, to date no synthetic route to this ingenious biological catalyst has been developed.
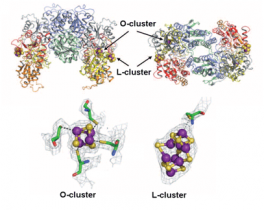
In order to gain insight into the mechanism of biosynthesis of the FeMoco, the structure of the protein responsible for the last step of cluster maturation, and of inserting the fully assembled cluster into nitrogenase, has been determined. This protein – called NifEN – has been crystallized in a state before cluster maturation, harboring an all-iron precursor form of the FeMoco. The crystal structure of Av-NifEN could be determined to a nominal resolution of 2.6 Å using data collected at SSRL beam line 12-2. Unexpectedly, the highly oxygen-sensitive co-factor precursor – named L-cluster – is attached on the periphery of the protein, exposed to the surrounding environment. The electron density of this cluster is highly reminiscent of the shape of the mature FeMoco, but the anomalous electron density suggests it to be composed of 8 iron atoms, instead of 7 irons and one molybdenum atom. (Anomalous electron densities are obtained when crystallographic experiments are conducted near absorption edges of elements, and basically provide element-specific electron densities.)
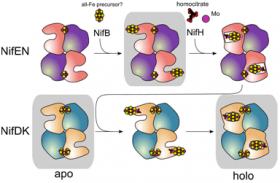
The protein itself was found in a conformation that resembled an average of the previously determined crystal structures of Av-NifDK in its mature form and in its cofactor-free form, respectively. Taking into account that these two proteins share about 30% sequence similarity, this observation suggests that the catalytic cycle of NifEN is an analogue of the maturation pathway of NifDK.
These results are one step in the direction of elucidating the mechanism of catalysis by nitrogenase, and may even enable synthetic chemists to explore different routes to chemically synthesize the FeMo cofactor.
Instrumental in this structure determination were the capabilities of SSRL beam line 12-2. The ability to screen many crystals (more than 200) robotically, together with the ability to vary the beam size and the high x-ray brightness at BL12-2 were crucial for the success of this project. It was possible to focus the beam on a small volume of a particular crystal, enabling measurement in small areas that were statistically more homogenous. Model building and interpretation was performed using about 20 datasets collected at 3 wavelengths on only 4 crystals.
Kaiser, J. T., Hu, Y., Wiig, J. A., Rees, D. C., and Ribbe, M. W. (2011) Structure of Precursor-Bound NifEN: A Nitrogenase FeMo Cofactor Maturase/Insertase,Science, 331, 91-94.

