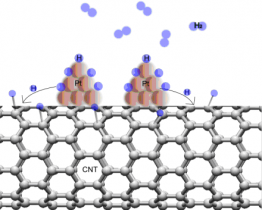
The proposal by Dillon et al.1 that carbon nanotubes (CNTs) could store large quantities of hydrogen by physisorption has led many groups to investigate the possibility of CNT-based hydrogen-storage materials. Theoretical predictions suggest that it is possible to achieve 100% hydrogenation of single-walled nanotubes (i.e., one hydrogen atom for every carbon atom) through a chemisorption mechanism in which stable C−H bonds are formed.2 As a result, carbon has been considered as an attractive option with its ability to store a high weight percentage of hydrogen. The mechanism of CNT hydrogenation would involve the breaking of the C−C π-bonds and the conversion of carbon atoms from sp2 to sp3 hybridization upon coordination with hydrogen. While previous studies have generated large concentrations of C−H bonds using atomic hydrogen sources, practical implementation of hydrogen storage in CNTs requires the development of low-barrier pathways for hydrogenation.
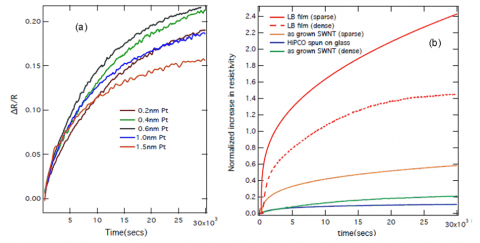
In this work, the concept of the “spillover” mechanism in heterogeneous catalysis was exploited to store hydrogen in carbon nanotubes. Researchers from Stanford University and SLAC National Accelerator Laboratory examined how the hydrogen uptake of single-walled carbon nanotubes (SWNTs) is influenced by the addition of a platinum nanoparticle catalyst. In-situ electrical conductivity measurements showed that the electrical conductivity of platinum-sputtered single-walled carbon nanotubes (Pt-SWNTs) decreased upon gaseous hydrogen exposure. Asteady increase in the resistance of the Pt-SWNTs during exposure to molecular hydrogen gas was observed. Figure 2a shows a typical resistance response for the Pt-SWNT composite samples under exposure to molecular hydrogen, with different amounts of deposited platinum. The rate of resistance change depended on catalyst particle size (figure 2a) as well as nature of the SWNTs (figure 2b). It was found that composites with maximum density of platinum nanoparticles showed the highest resistance change.
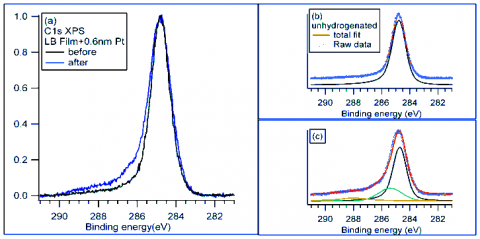
Ex-situcarbon 1s x-ray photoelectron spectroscopy (XPS) measurements were performed at SSRL Beam Line 13-2 to understand the origin of the resistance increase.After exposure to hydrogen at a pressure of 8.27 bar for 6 hours at room temperature, significant changes in the peak shape of the carbon 1s spectra were observed in the high-binding-energy region: in addition to the previously observed peak associated with sp-hybridized carbon, a new feature appears at 0.85 eV higher energy. This new spectral contribution is assigned to carbon atoms that have undergone rehybridization from sp2 to sp3 due to the breaking of C−C π-bonds and C−H bond formation3 and seen as direct evidence for the proposed spillover effect. Moreover, XPS measurements confirm that up to 16 ± 1.5 atomic percent of the carbon atoms undergo hydrogenation in the dispersed Langmuir−Blodgett (LB) films. LB films yield the highest hydrogenation capacity. Since the preparation method unbundles the SWNTs,4 these have higher surface area in contact with the platinum nanoparticles.
XPS and conductivity measurements indicate that the Pt-SWNTs store hydrogen by means of chemisorption, i.e. covalent C–H bond formation, which supports a hydrogenation mechanism, facilitated by “spill-over” of dissociated hydrogen from the platinum nanoparticles. It is also evident that for high storage capacity, samples with high catalyst particle density and SWNT surface area are required.
This work was supported by the Global Climate and Energy Project operated by Stanford University and carried out at the Stanford Synchrotron Radiation Lightsource, a National User Facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
[1] A. C. Dillon, K. M. Jones, T. A. Bekkedahl, C. H. Kiang, D. S. Bethune, and M. J. Heben, Nature (London) 1997, 386, 377-379.
[2]S. Park, D. Srivastava, and K. Cho, Nano Lett.2003, 3(9), 1273–1277.
[3]A. Nikitin, H.Ogasawara, D. Mann, R. Denecke, Z. Zhang, H. Dai, A. Nilsson, Phys Rev Lett. 2005, 95,225507.
[4] X. Li, L. Zhang, X. Wang, I. Shimoyama, X. Sun, W-S. Seo, and H. Dai, J. Am. Chem. Soc. 2007, 129, 4890-4891.
Bhowmick, R., Rajasekaran, S., Friebel, D., Beasley, C., Jiao, L., Ogasawara, H., Dai, H., Clemens, B., Nilsson, A.,“Hydrogen Spillover in Pt-Single-Walled Carbon Nanotube Composites: Formation of Stable C−H Bonds” J. Am. Chem. Soc., 133(14), pp 5580–5586, 2011(doi: 10.1021/ja200403m)

