Dioxygen is essential in life processes, and metalloenzymes activate dioxygen (O2) to carry out a variety of biological reactions including biotransformation of naturally occurring molecules and oxidative metabolism of xenobiotics. One primary goal in biomimetic research is to understand the mechanistic details of dioxygen activation and oxygenation reactions and the structures of reactive intermediates formed at the active sites of the metalloenzymes.
A group of Ewha Womans University researchers, led by Prof. Wonwoo Nam, recently prepared a series of metal-O2 intermediates as models of biological mononuclear active centers, which demonstrated biolgocially relevant small molecule catalysis. In biological systems, the metal site is held in place usually by protein-based ligands. O2 can then approach and bind to the metal center for transfer to target areas or for activation for reactions with substrates. The protein-based ligands play a crucial role in stabilizing the metal center and the nature, type and number of these ligands can affect the metal-O2 interaction strongly. The metal-O2 systems prepared by Nam et al. have cyclic ligand systems with differing ring sizes. Although this difference is small, the effect of ring size on the metal-O2bonding appeared to be dramatic. In collaboration with researchers at Stanford University and SSRL, Nam et al. investigated the role of the ligand ring size in affecting the geometric and electronic factors that determine the final structures of these metal-O2 complexes. They studied Ni-O2 and Co-O2 complexes with 12-membered (12-TMC) and 14-membered (14-TMC) (Figure 1) ring ligands using x-ray absorption spectroscopy (XAS) and extended x-ray absorption fine structure (EXAFS) data collected on the two Structural Molecular Biology Beam Lines 7-3 and 9-3 at SSRL and combined it with density functional theory (DFT) calculations.
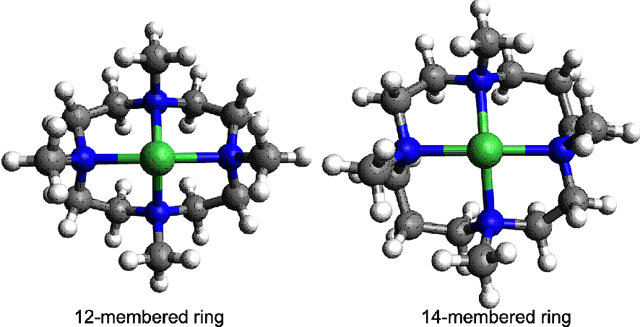
The study revealed that in the case of the O2 bound Ni compounds, [Ni(12-TMC)-O2]+ was a side-on bound Ni(III)-peroxide species, while the [Ni(14-TMC)-O2]+ was an end-on bound Ni(II)-superoxide species. This difference in electronic and geometric structures was assigned on the basis of Ni K-edge XAS and EXAFS experiments (Figure 2). DFT calculations showed that these differences arise due to the differences in ring size. The data show that the 12-TMC ligand in [Ni(12-TMC)-O2]+ is constrained to have an open face that can accommodate a side-on bound O2 while the 14-TMC is more planar and can only accommodate O2 bound in an end-on fashion.
![Figure 2 The normalized Ni K-edge XAS (inset shows the expanded pre-edge region) (left), EXAFS and their corresponding Fourier transforms (right) of [Ni(12-TMC)O2]+ and [Ni(14-TMC)O2]+. The shift in the edge energy positions and the difference in the EXAFS and Fourier transforms clearly indicate the differences in geometric and electronic structure. Figure 2.](/content/sites/default/files/images/science/highlights/2011/biomimetic-fig2-20110328.png)
A striking difference was observed in the comparison of the [Co(12-TMC)-O2]+ and [Co(14-TMC)-O2]+ systems. Both molecules have side-on bound Co(III)-peroxide electronic structures. The change in ring size did not affect the mode of binding or the redox state of the Co-O2 moiety. However, the EXAFS reveal that the [Co(14-TMC)-O2]+ molecule undergoes significant distortion to accommodate the side-on binding of the O2 moiety (Figure 3). DFT calculations reveal that as a six-coordinate molecule [Co(14-TMC)-O2]+, the stability of the Co(III) redox state over the Co(II) redox state wins over the sterics posed by the 14-TMC ring, leading to significant distortion in the ring to stabilize a Co(III) redox state. This results in a change in the spin state of the molecule; [Co(12-TMC)-O2]+ is S = 0, while [Co(14-TMC)-O2]+ is S = 1.
![Figure 3 The normalized Co K-edge XAS (inset shows the expanded pre-edge region) (left), EXAFS and their corresponding Fourier transforms (right) of [Co(12-TMC)-O2]+ and [Co(14-TMC)-O2]+. The similarity of the edge energy positions and the dramatic decrease in the EXAFS and Fourier transforms clearly indicate that the Co is Co(III) in both species, but the ring has undergone significant rearrangement. Figure 3.](/content/sites/default/files/images/science/highlights/2011/biomimetic-fig3-20110328.png)
The study showed that there is interplay of different factors including the relative stability of the metal site and the ligand sterics and electronics in determining the final mode of binding of O2 to the metal center. Importantly, the study indicates that the binding of O2 to biological metal centers is a complex combination of factors and each metal-ligand system should be individually evaluated for the type of metal-O2 interaction in order to evaluate O2 binding and activation. This study sheds light on two important metal centers and their coordination environments relevant to the reactivity of the metal-O2 intermediates. These studies on metal-O2intermediates have implications not only in the biological systems where metalloenzymes activate O2 for reactivity but also in organic synthesis where these compounds can be used as oxidizing agents or as better industrial catalysts. These studies have been reported in three publications in Nature Chemistry, Journal of the American Chemical Society andInorganic Chemistry.
SSRL operations are funded by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology program is supported by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and Department of Energy, Office of Biological and Environmental Research. The research was supported by NRF/MEST of Korea through the CRI and WCU (R31-2008-000-10010-0) Programs (to W.N.) and by the NIH DK31450 (to E.I.S).
(1) Sarangi, R.; Cho, J.; Nam, W.; Solomon, E. I. Inorg. Chem. 2011, 50, 614.
(2) Cho, J.; Sarangi, R.; Kang, H.; Lee, J.; Kubo, M.; Ogura, T.; Solomon, E. I.; Nam, W. J. Am.
Chem. Soc. 2010, 132, 16977-16986.
(3) Cho, J.; Sarangi, R.; Annaraj, J.; Sung Yeon, K.; Kubo, M.; Ogura, T.; Solomon, E. I.; Nam,
W. Nat. Chem. 2009, 1, 568-572.
These studies have been reported in three publications in Nature Chemistry, Journal of the American Chemical Society and Inorganic Chemistry

