Nephrogenic systemic fibrosis (NSF) is an incurable, debilitating, and potentially fatal disease found exclusively in patients with decreased kidney function that comprises of a fibrosing disorder of the skin and systemic tissues. Patients develop lesions where the skin becomes thickened, swollen and woody in texture. Patients can also become severely disabled, experiencing muscle weakness, joint contractures (shortening of tendons), and bone pain. The clinical course is progressive in most cases and there is no consistently effective treatment.
The disease is associated with exposure to gadolinium based contrast agents (GBCA) used in magnetic resonance imaging (MRI), however there has been no direct evidence of a casual link. This class of contrast agent is commonly used to enhance the visibility of blood vessels and so, for example, they are useful in visualizing lesions and tumors. GBCAs exploit the large magnetic moment and long electronic relaxation time of the paramagnetic Gd3+ ion with its S=7/2 electron spin. Since Gd is toxic, the GBCAs comprise Gd3+bound to a chelator which is designed to both solubilize the Gd and make it biologically inaccessible. GBCAs are normally rapidly eliminated intact by the kidneys. More than 20 million MRI procedures are performed annually, with 25-30% of these examinations involving contrast agents, and sales of these products constitute a billion dollar market.
Tissues samples from many patients with NSF have previously been shown to contain micron sized, insoluble Gd containing deposits, however the significance of these deposits has been an intractable biomedical problem. As GBCAs are the only significant source of human Gd exposure it is very likely that the origin of the Gd in these particles are GBCAs administered during an MRI procedure. This raises the question: has the GBCA exposure has caused the NSF disease or has an already existing disorder caused the GBCAs to be precipitated? In order to answer this question it is important to understand the chemical nature of these tissue deposits. If they are precipitated intact GBCAs then they may well be adventitious products of an already existing illness. On the other hand, if the Gd ions are chemically modified then this means they have been released from the GBCA chelator, which in turns implies a possible causal link between GBCA exposure and the disease.
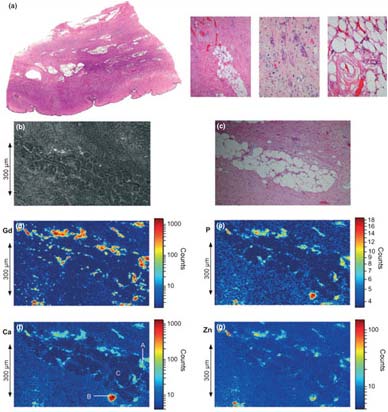
In this study, synchrotron based x-ray fluorescence (SXRF) microscopy and extended x-ray absorption fine structure (EXAFS) spectroscopy were utilized to characterize the Gd deposits in skin from a patient with NSF. Figure 1 shows a summary of the microprobe results. The tissue itself shows typical histopathology of NSF, showing subcutaneous fat lobules surrounded by fibrotic bands. The SXRF images show Gd deposited throughout the tissue, and especially surrounding the fat lobule. The maximum observed concentration of Gd was 0.41 g cm-3 with an average Gd concentration of 0.011 g cm-3. The SXRF images also show a clear association of Gd with Ca and P. Throughout the Gd rich areas, the x-ray analyses indicate a consistent ratio of Gd:Ca:P of 1.0:0.43:1.6.
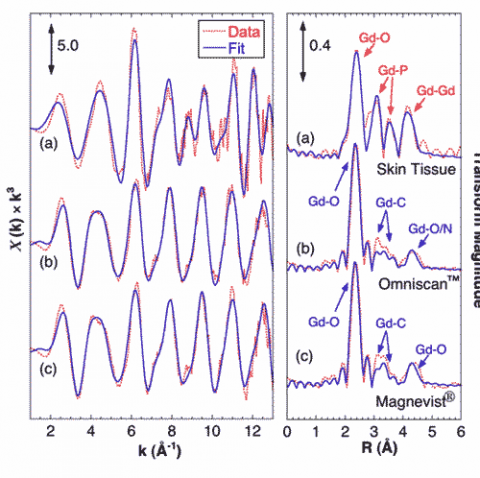
Gd EXAFS from the skin sample shows a very different coordination environment from that of the GBCAs, and fitting analysis shows that the tissue-bound Gd is coordinated by phosphate (Figure 2). EXAFS from both GBCAs show that Gd is coordinated in the diethylene triamine pentaacetic acid (DTPA) derivative structure, consisting of O atoms at 2.4 Å, a complex set of C atoms around 3.5 Å, and a split peak of outer O/N atoms at 4.2 – 4.5 Å. The Gd tissue sample shows a very different structure with O atoms at 2.39 Å, P at 3.11 and 3.72 Å, and Gd neighbors at an average of 4.05 Å. These structural parameters are consistent with structures of hydrated Gd-phosphates and mixed metal salts such as KCaGd(PO4).
These results provide the first atomic scale constraints on the molecular structure of Gd deposits in NSF tissue samples, as well as the first direct evidence for the chemical release of Gd from GBCA in human tissue. SXRF shows a strong, nearly invariant correlation between Gd, P, and Ca. EXAFS clearly shows Gd present in a GdPO4-like structure, with little to no Gd still observed in the original GBCA. It seems reasonable to suppose that these deposits in NSF are either causally related to the disease or at least represent a marker of the release of Gd from the chelated GBCA. This in turn helps identify the challenges to chemists and physicians hoping to treat NSF patients.
Simon. J. George, Samuel. M. Webb, Jerrold. L. Abraham, Stephen P. J. Cramer, “Synchrotron X-ray Analysis Demonstrate Phosphate-Bound Gadolinium in Skin in Nephrogenic Systemic Fibrosis”, British Journal of Dermatology, 163, 1077 (2010)

