The cellular export of toxins and substrates is a fundamental life process, and members of the MATE family are the last class of MDR transporters to be structurally characterized. MATE transporters are involved in a variety of important biological functions across all kingdoms of life. In plants, MATE transporters are highly prevalent, with 58 paralogues found in Arabidopsis thaliana, and they secrete a diverse range of secondary metabolites as a defense against herbivores and microbial pathogens1. In addition, plant MATE transporters are important in tolerance towards phytotoxic aluminium in acidic soils, a major limitation of crop production in 50% of the world’s arable land2. In mammals they export a structurally diverse array of xenobiotic cations in the liver and kidney, influencing the plasma concentrations of many drugs, including metformin, a widely prescribed type 2 diabetes medication, thereby mitigating therapeutic efficacy. Bacterial MATE transporters function primarily as xenobiotic efflux pumps and can confer resistance to tigecycline, a new glycylcycline-class antibiotic developed to overcome methicillin-resistant and vancomycin-resistant Staphylococcus aureus. MATE transporters use either H+ or Na+gradients across the membrane to drive substrate export, although the coupling mechanism is not well understood.
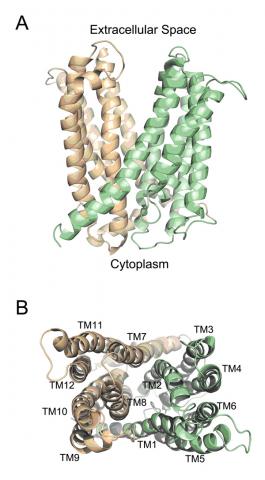
A team of researchers led by Prof. Geoffrey Chang at The Scripps Research Institute determined the structure of the MATE transporter NorM from Vibrio cholarae to 3.65 Å resolution (Fig. 1). They used resources at SSRL, CLS, ALS, and APS to collect the single crystal diffraction data for the work. The NorM transporter is responsible for widespread resistance to ciprofloxacin and other fluoroquinolones (a broad-spectrum, inexpensive class of antibiotics) and to tigecycline, a new class of drug specifically designed to overcome that antibiotic resistance.
This structure of a bacterial MATE transporter reveals an outward-facing conformation with two portals open to the outer leaflet of the membrane and a unique topology of the predicted 12 transmembrane helices distinct from any other known MDR transporter. The authors also report a cation-binding site in close proximity to residues previously deemed critical for transport. This conformation probably represents a stage of the transport cycle with high affinity for monovalent cations and low affinity for substrates. The structure of NorM reveals the last known MDR transporter family revealed by X-ray crystallography. The shared V-shaped conformations among the MDR transporter family suggest that this may be providing a molecular basis for hydrophobic/amphipathic substrate transport.
1. Morita, M. et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum . Proc. Natl Acad. Sci. USA 106, 2447–2452 (2009)
2. Wood, S., Sebastian, K. & Scherr, S. J. Pilot Analysis of Global Ecosystems: Agroecosystems Vol. 12 (World Resources Institute, 2000)
Xiao He, Paul Szewczyk, Andrey Karyakin, Mariah Evin, Wen-Xu Hong, Qinghai Zhang& Geoffrey Chang. Nature, 467, 991-994 (2010)