Targeting of newly synthesized membrane proteins to the endoplasmic reticulum (ER) is an important cellular process. Most membrane proteins are recognized and targeted co-translationally by the signal recognition particle (SRP). A number of membrane proteins (eg. SNAREs, apoptosis factors, and protein translocation components) are 'tail-anchored' by a single carboxy-terminal transmembrane domain. Due to this topological constraint, these proteins are not able to follow the SRP-dependent co-translational pathway that typifies most integral membrane proteins. Instead, these proteins must find their correct membrane for insertion post-translationally (1). The ATPase Get3 was the first protein identified directly involved in TA targeting and is part of the Get pathway (Guided Entry of Tail-anchored proteins) that also contains the ER membrane proteins Get1/2 and the putative ribosome receptor proteins Get4/5 (2). Multiple studies have shown that Get3 binds directly to the hydrophobic tail-anchors and, in conjunction with ribosome and ER factors, utilizes an ATP cycle to bind and then release TA proteins at the ER membrane.
To get a detailed mechanistic view of how Get3 performs its important targeting function a team at Caltech led by Bil Clemons used data collected at SSRL Beam Line 12-2 to determine the three-dimensional structure of Get3/TRC40 (Fig. 1). The structure of Get3 in the ADP form was a hexamer (Fig. 1a). It is expected that Get3 functions as a dimer (Fig. 1b). Based on homology to other proteins, the structure allowed for the prediction that binding of ATP would lead to a dramatic conformational change bringing two hydrophobic grooves together to form a TA-binding pocket. The team probed functional interfaces and essential residues using phenotypic rescue allowing them to define a model of how Get3 couples ATP hydrolysis to the binding and release of TA-proteins.
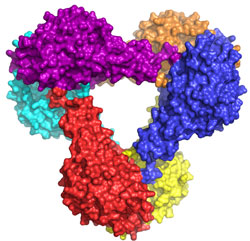
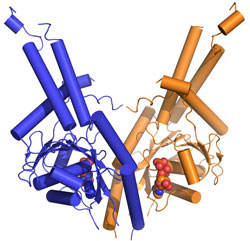
This work represents the first structural study in this novel pathway. Future work will allow for a refining of this model including the details of the binding of substrate and activation for ATP hydrolysis.
- Kutay U, Hartmann E, Rapoport TA (1993) A class of membrane proteins with a C-terminal anchor. Trends Cell Biol 3:72-75.
- Schuldiner M, et al. (2005) Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell 123:507-519.
Suloway, C. J. M., Charton, J. W., Zaslaver, M., and Clemons, Jr. W. M. (2009) Model for eukaryotic tail-anchored protein binding based on the structure of Get3. PNAS, 106: 14849-14854.




