Signals originating at the cell surface are conveyed by a complex system of interconnected signaling pathways to the nucleus. They converge at transcription factors, which in turn regulate the transcription of sets of genes that result in the gene expression. Many human diseases are caused by dysregulated gene expression and the oversupply of transcription factors may be required for the growth and metastatic behavior of human cancers. Cell permeable small molecules that can be programmed to disrupt transcription factor-DNA interfaces could silence aberrant gene expression pathways. Pyrrole-imidazole polyamides are DNA minor groove binding small molecules that are programmable for a large repertoire of DNA motifs.
Py/Im polyamides bind the minor groove of DNA sequence specifically (1), encoded by side-by-side arrangements of N-methylpyrrole (Py) and N-methylimidazole (Im) carboxamide monomers. They have been shown to permeate cell membranes (2), access chromatin, and disrupt protein-DNA interactions. X-ray crystallography of antiparallel 2:1 binding polyamides in complex with DNA reveal a 1-2 Å widening of the minor groove (3). This modest structural perturbation to the DNA helix by the side-by-side stacked arrangement of aromatic rings does not explain the large number of transcription factor-DNA interfaces disrupted by minor groove binding hairpin Py/Im polyamides. It must be that the turn unit in the hairpin oligomer connecting the 2 antiparallel strands plays a structural role.
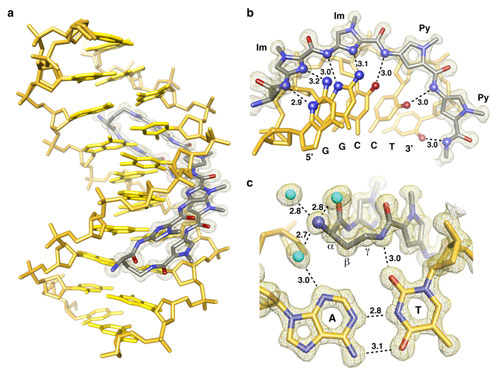
Using data collected at SSRL Beam Line 12-2, a research team led by Peter Dervan from California Institute of Technology determined the three dimensional high resolution an 8-ring cyclic Py/Im polyamide bound to the central 6 bp of the sequence d(5'-CCAGGCCTGG-3')2 (Fig. 1). The structure reveals a 4 Å widening of the minor groove and compression of the major groove along with a >18° bend in the helix axis toward the major groove. This allosteric perturbation of the DNA helix provides a molecular basis for disruption of transcription factor-DNA interfaces by small molecules, a minimum step in chemical control of gene networks. Allosteric control over transcription factor regulatory networks by small molecules that bind distinct locations on promoter DNA provides a mechanism for inhibiting excess transcription factor activity.
- Dervan PB (2001) Molecular recognition of DNA by small molecules. Bioorg Med Chem. 9:2215-2235.
- Edelson BS, et al. (2004) Influence of structural variation on nuclear localization of DNA-binding polyamide-fluorophore conjugates. Nucleic Acids Res 32:2802-2818.
- Kielkopf CL, Baird EE, Dervan PB, Rees DC (1998) Structural basis for G.C recognition in the DNA minor groove. Nat Struct Biol 5:104-109.
Chenoweth, D. M. and Dervan. P. B. (2009) Allosteric modulation of DNA by small molecules PNAS, 106:13175-13179.




