
Iron is one of the most important elements to life. Despite its paramount importance and relative abundance, dissolved iron concentrations are often very low, in part due to the formation of very stable iron minerals in most oxidizing environments. Since soluble iron is available to living organisms, iron deficiencies are widespread, and the factors that influence iron solubility are important to understand ecosystem function. Perhaps nowhere is iron deficiency more apparent than in the world's oceans, where as much as 30-40% of the ocean is affected by iron limitation due in part to the limited supply of dissolved iron to these areas. Photosynthesis is particularly dependent on iron availability, in part due to the high iron requirements needed to produce chlorophyll.
External supplies of iron to the world's oceans come largely from fine sediments and dusts that can be transported from continents by ocean currents, or as aerosols deposited on the ocean surface. Aerosols are most notably derived from arid and seasonally-arid areas, where winds can entrain significant particulates and transport them long distances. Glaciers also produce considerable sediments that can form aerosols; Alaska contains numerous glaciers that efficiently grind rock that delivers both aerosols and suspended sediments to the Gulf of Alaska (Figure 1). Anthropogenic aerosols from fossil fuel emissions and other sources are can be important in some areas.
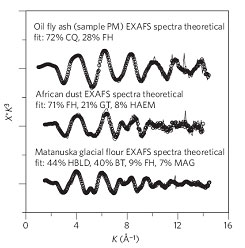
Aerosols often represent a majority of iron reaching the surface of the ocean, yet relatively little is known about the processes that control their solubility and fate in the environment. What is apparent is that aerosol source regions can have widely different iron solubilities (up to three orders of magnitude), and that differences in solubility are not simply a function of iron concentration in the aerosol. The variable solubility of iron particulates is a function of iron speciation, which is effectively measured using X-ray absorption spectroscopy. Dusts derived from different locations have different oxidation states, bonding environments and mineralogies, with each phase having a distinct solubility (Figure 2). The fraction of soluble iron is highest in aerosols from the fossil fuel combustion (oil fly ash). These materials contain predominantly ferric iron, Fe(III), which is normally relatively insoluble, but in this case, the iron is bound in sparingly soluble ferric sulfates. Aerosols derived from the Saharan desert contain abundant insoluble iron(III) oxides, including ferrihydrite, hematite and goethite, most of which do not dissolve in ocean water. Glacial rock flour has intermediate solubility, with solubility depending on the content of relatively reactive mixed valence silicates derived from the physical weathering of rocks beneath the glaciers.
This work has three important implications to our understanding of iron cycling in the ocean. This work provides a chemical basis for the substantial differences in iron solubility for different iron particles. Second, it shows that glacial processes can affect the ocean in complex and previously unexplored ways. As glaciers recede in response to global climate change, the increased dust delivery to the oceans may serve as a feedback mechanism to influence atmospheric carbon dioxide levels and thereby moderate climate change. At very least, changes in climate will affect the distribution and source of dust sources to the world's oceans, thereby affecting marine productivity and the global carbon cycle. Third, this work highlights the potentially important role of anthropogenic aerosols in regulating dissolved iron levels, and thus photosynthesis, in the oceans. In the North Pacific Ocean, the increased fossil fuel use in China and elsewhere in Asia may increase the aerosol concentration of oil fly ash. Since oil fly ash is highly soluble, this increase could profoundly affect primary production.
For more on the role of iron in the oceans, see the following: (a) Martin, J. H., Gordon, R. M. & Fitzwater, S. E. Limnol. Oceanogr. 36, 1793-1802 (1991).; (b) Moore, J. K., Doney, S. C., Glover, D. M. & Fung, I. Y. Deep-Sea Res. I 49, 463-507 (2002). (c) Jickells, T. D. et al. Science 308, 67-71 (2005). (d) Lam, P. J. & Bishop, J. K. B. Geophys. Res. Lett. 35, L07608 (2008). (e) Journet, E., Desboeufs, K. V., Caquineau, S. & Colin, J. L. Geophys. Res. Lett. 35, L07805 (2008).
A. W. Schroth, J. Crusius, E. R. Sholkovitz and B. C. Bostick, "Iron Solubility Driven by Speciation in Dust Sources to the Ocean", Nature Geosci. 2, 337 (2009)

