Sulfur is essential for life. It plays important roles in the amino acids methionine and cysteine, and has a structural function in disulfide bonds. As a component of iron-sulfur clusters it takes part in electron and sulfur transfer reactions.1 Glutathione, a sulfur-containing tripeptide, is an important part of biological antioxidant systems.2 Another example for the biological relevance of sulfur is the amino acid taurine, which is present in high concentrations in algae and the animal kingdom. Taurine has been implicated in a range of physiological phenomena, but its osmolytic role in cell volume regulation has been studied in greatest detail.3
In situ information on sulfur is rare despite its important biological role. This is due to the fact that sulfur is not easily accessible with most biophysical techniques. In recent years, sulfur x-ray absorption spectroscopy (XAS) has become increasingly important in the study of sulfur species in biological systems.4 The near-edge region of the XAS spectrum is a sensitive probe of electronic structure and hence chemical form.5
Near-edge spectra of sulfur compounds are particularly diverse, with a chemical shift range of about 14 eV.6 The technique has been used to analyze complex mixtures by fitting experimental sulfur spectra as linear combinations of model spectra, effectively providing an in situ measurement of all the sulfur species that are present, the sulfur metabolome.6
Gnida & Yu Sneeden et al. used sulfur XAS for an in situ study of taurine uptake into living mammalian cell cultures. Madin-Darby canine kidney (MDCK) cell cultures were cultured on polycarbonate membranes on which the cells develop into a polarized monolayer (Figure 1), exhibiting characteristics of kidney distal tubules.7,8
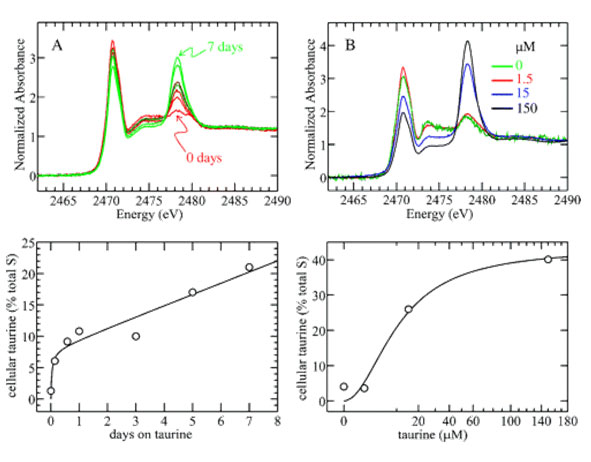
Figure 2 (upper row) displays the sulfur spectra of such intact MDCK monolayers, which were maintained in growth medium containing taurine either (A) for different amounts of time or (B) in different concentrations. Taurine, a sulfonate RSO3-, gives rise to a char acteristic peak at 2478 eV in the spectrum and its contribution to the total cellular sulfur signal is determined by curve-fitting.6 Plotting cellular taurine fractions as a function of (A) taurine incubation times or (B) taurine concentrations yields time- and dose-dependent uptake curves, respectively (Figure 2, lower row). Both curves clearly exhibit saturation behavior. Note that the amount of cellular taurine can become considerable. In cells, cultured for 6 days in growth medium containing 150 mM taurine, a cellular taurine content of 40% has been determined (Figure 2B).
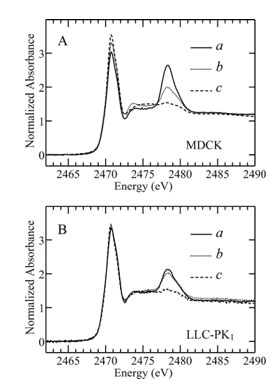
Another part of the study addressed the polarity of taurine uptake. The two cell surfaces (basolateral and apical, corresponding to the blood vessel and the urinary tubule respectively) of polarized renal epithelial monolayers (such as MDCK) are not identical and the taurine uptake behavior has been reported to depend on the cell surface.9 Two different cell lines were compared in the present study, MDCK cells and Lewis lung carcinoma pig kidney LLC-PK1 cells (Figure 3A and 3B, respectively). Cells were kept in taurine-free medium until one day before the experiment, when 50 mM taurine were added to either the basolateral (Figure 3, curve a) or the apical (Figure 3, curve b) medium. Taurine is taken up on both surfaces of MDCK monolayers but more by the basolateral surface. In contrast, the fractional taurine signals in LLC-PK1 cells are comparable whether taurine is present in the basolateral or apical medium. Also, accumulation levels in LLC-PK1 cells correspond to levels reached in MDCK cells via apical uptake. Obviously, MDCK cells are able to accumulate much larger amounts of taurine, an ability that is primarily mediated by taurine transport on the basolateral surface. This observation of the polarity of taurine uptake agrees well with previous studies using radioactive tracer methods.9 MDCK and LLC-PK1 cells originate from different parts of the kidney tubule, and taurine transport is thus expected to differ in that proximal cells (LLC-PK1) should reabsorb filtered taurine while distal cells (MDCK) need to regulate cell volume in response to osmotic stress and use taurine for this purpose.
In conclusion, sulfur XAS has been demonstrated to be a valuable and potentially nonde structive tool for studying the sulfur metabolome of intact, living mammalian tissue. Previous taurine uptake and release studies have typically been performed using radioactive tracer methods.9 However, measuring tracer counts requires destruction of the cell monolayer. Also, from the measurement of radiolabel alone, one cannot be sure of the direction of mass transport. In contrast, sulfur XAS allowed monitoring taurine uptake in situ.
- Beinert, H. (2000) Eur. J. Biochem. 267, 5657-5664.
- Schafer, F. Q., and Buettner, G. R. (2001) Free Radical Biol. Med. 30, 1191-1212.
- Huxtable, R. J. (1992) Physiol. Rev. 72, 101-163.
- Akabayov, B., Doonan, C. J., Pickering, I. J., George, G. N., and Sagi, I. (2005) J. Synchrotron Radiat. 12, 392-401.
- George, G. N., Hedman, B., and Hodgson, K. O. (1998) Nat. Struct. Biol. 5, 645-647.
- Pickering, I. J., Prince, R. C., Divers, T., and George, G. N. (1998) FEBS Lett. 441, 11-14.
- Taken from http://www.mpi-magdeburg.mpg.de/research/groups/bpt/. Reproduction with kind permission from Prof. Udo Reichl.
- Rindler, M. J., Chuman, L. M., Shaffer, L., and Saier, M. H. (1979) J. Cell Biol. 81, 635-648.
- Jones, D. P., Miller, L. A., and Chesney, R. W. (1993) Am. J. Physiol. 265, F137-F145.
Gnida, M., Yu Sneeden, E., Whitin, J. C., Prince, R. C., Pickering, I. J., Korbas, M., George, G. N. "Sulfur X-ray Absorption Spectroscopy of Living Mammalian Cells: An Enabling Tool for Sulfur Metabolomics. In Situ Observation of Uptake of Taurine into MDCK Cells", 2007, Biochemistry, 46, 14735-14741.




